Introduction
Fluted scales or iceryine scale insects belong to the tribe Iceryini within the family Monophlebidae (Hemiptera: Coccomorpha) (Unruh & Gullan, 2008). There are 88 species of iceryine scale insects in five genera: Crypticerya Cockerell (28 spp.), Gigantococcus Pesson and Bielenin (19 spp.), Icerya Signoret (38 spp.), Echinicerya Morrison (1 sp.), and Gueriniella Fernald (2 spp.) (García Morales et al., 2022). Iceryine scale insects include many polyphagous species. Human activities have introduced species of Crypticerya and Icerya to new habitats where some of them have become invasive. In South America, several polyphagous species, i.e., Crypticerya brasiliensis (Hempel), C. genistae (Hempel), C. montserratensis (Riley & Howard), C. multicicatrices Kondo & Unruh, C. palmeri (Riley & Howard), C. zeteki (Cockerell), Icerya purchasi Maskell, and I. seychellarum (Westwood) have been reported to cause damage to plant species of economic importance (Kondo et al., 2012; Kondo, Gullan, et al., 2016; Kondo, Ramos-Portilla, et al., 2016; Ramos et al., 2017). The damage caused by fluted scales is divided into direct damage, consisting of the loss of water and nutrients in plant sap due to scale feeding activity, and indirect damage due to the development of sooty mold fungi on the honeydew deposits produced by these insects (Kondo, Becerra et al., 2014).
In June 2021, the first author detected infestations by two unknown species of Crypticerya in several urban green areas in Guayaquil city (Guayas Province, Ecuadorian Coast). However, the identities and infestation levels of these alien species remained unconfirmed. The main goal of this study is to identify the two unknown invasive fluted scales at the species level. Furthermore, a list of host plants infested by each of the two species, with an estimated infestation level on the host by each species, is provided. Finally, some suggestions on the possible management of these invasive species are discussed.
Materials and methods
Study area
The study took place in 29 urban green areas in three cities in Guayas, a province located on the Ecuadorian Coast: Guayaquil city (79°52′W, 02°10′S, ca. 11 m altitude), Samborondón (79°43′W, 01°57′S, ca. 23 m altitude), and Daule (79°58′W, 01°52′S, ca. 9 m altitude). The locations of the urban green areas sampled are shown in Figure 1. The map of the studied area was prepared using ArcGIS (version 10.4). The climate in Guayas is characterized by a mean annual rainfall of 1,030 mm, 85% mean relative humidity, and 26 °C mean annual temperature (Instituto Oceanográfico y Antártico de la Armada [INOCAR], 2021. Usually, a well-defined wet season occurs from January to April, while the dry season occurs from May to December (INOCAR, 2021). The predominant natural vegetation is tropical dry forest, mangroves, and semi-arid shrubland. Urban green areas in the cities of Guayas are characterized by the presence of both alien and native plant species, mainly of the families Fabaceae, Malvaceae, Bignoniaceae, Meliaceae, Burseraceae, and Rubiaceae (Molina-Moreira et al., 2015).

Source: Elaborated by the authors using ArcGIS (version 10.4).
Figure 1. Study area and sampling points to determine infestation levels and host plants of two Crypticerya species. a. A map showing the location of Guayas in Ecuador (top left, inset) and the study area in Guayas, Ecuador (red rectangle). b. A larger-scale map showing the locations of the sample sites in Guayaquil, Samborondón, and Daule.
Taxonomic identification
For each host plant, about 25-30 individuals of the fluted scale species in different developmental stages were collected from urban green areas in Guayaquil, Daule, and Samborondón. The samples were collected into plastic bags and transported to the laboratory. The specimens were preserved in 70% ethanol. For species identification, some adult female specimens of each species were slide-mounted using the method of Sirisena et al. (2013), with a single specimen on each slide. Voucher specimens are deposited in the Entomological Collection of the Agrocalidad Laboratory, Manta, Ecuador.
Species were identified using the taxonomic keys by Kondo et al. (2012) and Kondo, Gullan, et al. (2016), resulting in the identification of two species. The identifications were verified against the diagnoses of C. multicicatrices in Kondo & Unruh (2009) and Sotelo & Kondo (2016), and C. genistae in Mestre-Novoa et al. (2016) and Miller et al. (2014).
Identification of host plants
Most plant hosts were identified in situ with the mobile app Pl@ntnet (Goëau et al., 2013). Samples of host plants that could not be identified in situ were collected, taking, in some cases, a photographic record of flowers, fruit, stems, and leaves. Plant samples were compared with the descriptions and bibliography of Cisneros-Bohórquez & Molina-Moreira (2017), Palacios (2016a, b), and Molina-Moreira et al. (2015). Scientific names were verified using the database “The Missouri Botanical Garden’s VAST - Vascular Tropicos” (Tropicos, 2021). Plants were also recorded according to their growth habit, i.e., climbing plants, herbaceous plants, shrubs, trees, and palms.
Determination of infestation levels of fluted scales
Infestation levels of the two fluted scale species on the host plants were determined in situ from “snapshot” observations made by the authors in the field from July to October 2021. A simple way to assess the levels of infestation was used (Kondo et al., 2012): (i) No infestation: Zero individuals of the fluted scale present; (ii) Low: From one to several fluted scale individuals on leaves, branches, or other plant parts; (iii) Medium: High populations of fluted scales on leaves, branches, but without apparent symptoms of damage to the plant; and (iv) High: Leaves or branches highly infested, with symptoms of plant damage, e.g., sooty molds, yellowing of leaves, dried branches or dieback.
A Chi-square test and analysis of standardized residuals (s.r.) were used to analyze the data on infestation levels (low, medium, and high). Data analysis was carried out using the statistical software R run in R Studio (R Studio Team, 2019).
Results and discussion
The two fluted scale species recorded were found to belong to the species Crypticerya genistae (Hempel) and C. multicicatrices Kondo & Unruh; both are new records for Ecuador. According to Kondo, Gullan, et al. (2016), C. genistae is native to South America and probably originated in Brazil, where it was described over 100 years ago. The soybean fluted scale, C. genistae, has been reported from 17 countries in the New World: North America (Florida), Central America (Mexico), 13 Caribbean islands, and South America (Brazil and Colombia) (García Morales et al., 2022), and accidentally introduced to West Africa (countries not specified) (Malumphy, 2014).
The Colombian fluted scale, C. multicicatrices, is native to continental Colombia and, in 2010, was reported as invasive species on San Andrés and Old Providence Islands (Colombia) (Kondo et al., 2012; Kondo, Gullan et al. 2014). The species commonly infests branches and leaves of its hosts but may occur on the tree trunk and fruits when populations are high. Between 2010 and early 2013, damage caused by C. multicicatrices resulted in the loss of competitiveness and profitability in the agricultural sector of San Andrés Island, decreasing the quality of life of native families (Kondo, Becerra et al., 2014).
Host plants and infestation levels recorded
Crypticerya genistae
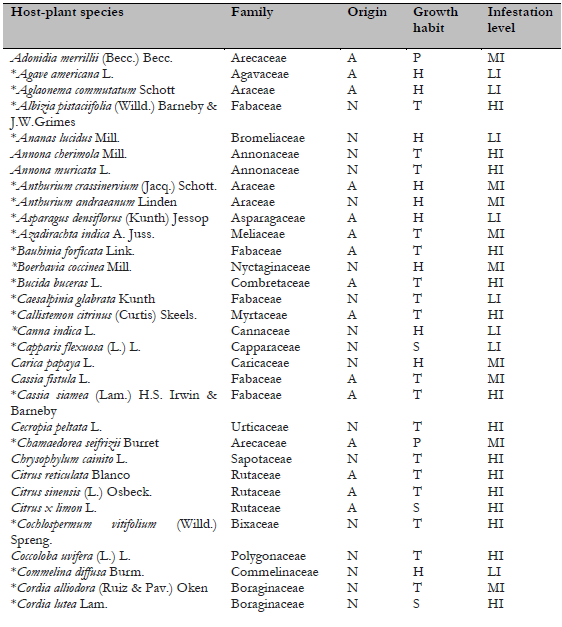
One hundred host-plant species belonging to 77 genera in 22 families have been reported as hosts for C. genistae (García Morales et al., 2022; Mestre-Novoa et al., 2016). In the present study, C. genistae was recorded on 13 host-plant species. Of these, eight belong to the family Fabaceae, the others to Asteraceae, Lamiaceae, Onagraceae, and Nyctaginaceae (Table 1). Eleven of these species are new host plant records for C. genistae. Among the host plants recorded in the study, eight species are native, whereas five species are alien to Ecuador (Table 1). Most of the recorded hosts of C. genistae are herbaceous (nine species), a few are trees (three species), and only one species is a shrub (Table 1). This result suggests that herbaceous plants are the preferred hosts for C. genistae.
Previous studies in Cuba, Florida (USA), and Guadeloupe in the Caribbean (France) reported that preferred hosts of C. genistae belong to the families Asteraceae, Euphorbiaceae, and, particularly, Fabaceae (Etienne & Matile-Ferrero, 2008; Hodges et al., 2008; Mestre-Novoa et al., 2016). Besides these host-plant families, the Malvaceae and Solanaceae also are common hosts of C. genistae (Kondo, Gullan, et al., 2016).
Table 1. Host plants of Crypticerya genistae recorded in Guayas, Ecuador
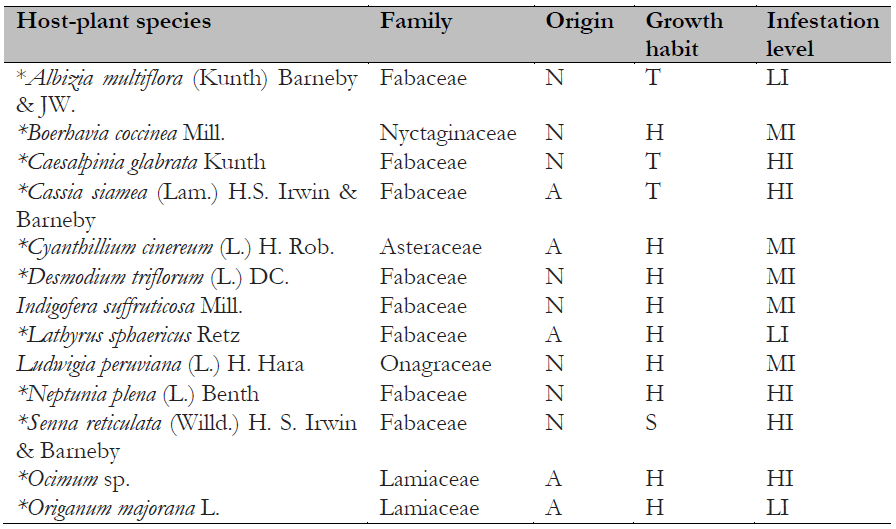
Note.Plant origin codes: A: Alien species; N: Native species. Growth habit codes: H: Herbaceous, S: Shrub, T: Tree. Infestation level codes: LI: Low Infestation, MI: Medium Infestation, HI: High Infestation. * = New host-plant record for C. genistae.
In Florida, where C. genistae is an alien invasive species, 47 hosts for this scale species have been reported (Hodges et al., 2008). In Cuba, C. genistae has been collected from 23 host species (Mestre-Novoa et al., 2016). This study found 11 new host-plant records for C. genistae (Table 1).
Native and alien plant species did not differ significantly in their infestation levels (X2 =1.73; p = 0.42; d.f. = 2), and infestation levels were not significantly different between growth habits either (X2 = 4.81; p = 0.30; d.f. = 4). Herbaceous species tended to have higher infestation levels compared to other growth habits. The host species with the highest levels of infestation were Caesalpinia glabrata, Cassia siamea, Neptunia plena, Senna reticulata (Fabaceae), and one undetermined species of Lamiaceae, Ocimum sp.
Some host plants (native and alien) with different levels of infestation by C. multicicatrices and C. genistae are shown in Figure 2.

Source: A-F and I by T. Kondo, G-H by M. Arias
Figure 2. Examples of levels of infestation (High: HI; Medium: MI; Low: LI) on some host species of C. multicicatrices and C. genistae. Host plants for C. multicicatrices: A. Tecoma castanifolia (HI); B.Adonidia merrillii (MI); C. Agave americana (LI). Host plants for C. genistae: D.Neptunia plena (HI); E. Desmodium triflorum (MI); F.Origanum majorana (LI). Host plants for both C. multicicatrices and C. genistae: G. Senna reticulata; H.Boerhavia coccinea; I.Ludwigia peruviana
Crypticerya multicicatrices
A total of 148 host-plant species belonging to 53 genera in 118 families have been reported as hosts for C. multicicatrices (García Morales et al., 2022; Sotelo & Kondo, 2016). In the present study, we identified 111 host-plant species belonging to 50 families as hosts of this species (Table 2). The families Fabaceae, Arecaceae, and Araceae had the most significant number of host-plant species, with 21 species (18.9%), ten species (9%), and seven species (6.3%), respectively. The other plant families had from one to four plant species each. Of the host plants, 54% (60 species) are of alien origin, whereas 46% (51 species) are native to Ecuador. Most hosts are trees (44%; 49 species), followed by herbaceous plants (34%; 38 species).
On San Andrés, Old Providence, and Santa Catalina Islands (Colombia), where C. multicicatrices is an alien invasive species, 147 host-plant species have been recorded for this scale insect (Kondo et al., 2012; Kondo, Gullan et al., 2014; Silva-Gómez et al., 2013). This study records 85 additional host-plant species for C. multicicatrices (Table 2). One of the studied locations was the Jardín Botánico de Guayaquil, which contains many alien plant species from different regions. In this location, C. multicicatrices was found infesting a wide variety of alien host plants, e.g., Mimusops coriaceae, native to Madagascar; Licania tomentosa, native to Brazil; Mespilus germanica, native to western Asia; and Pistacia vera and Streblus indicus, native to China.

Note.Plant origin codes: A: Alien species; N: Native species. Growth habit codes: C: Climbing plant, H: Herbaceous, P: Palm, S: Shrub, T: Tree. Infestation level codes: LI: Low Infestation, MI: Medium Infestation, HI: High Infestation. * = New host-plant record for C. multicicatrices.
Although the introduced tree Terminalia catappa is frequent in the green areas studied (I. Herrera, personal observation), C. multicicatrices does not infest this plant, which is consistent with observations on San Andrés Island by Kondo et al. (2012), who reported no incidence of C. multicicatrices on T. catappa. An in vitro experimental study conducted by Redo et al. (2019) determined that the water-ethanol fraction of T. catappa leaf possesses high biolarvicidal activity against third-instar larvae of Aedes aegypti. Studies that detect the effects of chemical components of T. catappa on C. multicicatrices are needed to elucidate the resistance mechanism of T. catappa against infestations by C. multicicatrices.
Of the total of host-plant species of C. multicicatrices detected, 47% (53 species) had high levels of infestation, 30.6% (34 species) had medium levels of infestation, and 21.6% (24 species) had low levels of infestation. No significant differences in the infestation levels between native and alien species (X2 = 1.49; p = 0.5; d.f. = 2) were found. The infestation level was significantly different between plant growth habits (X2 = 22.36; p = 0.004; d.f. = 8). Herbaceous plant species had a low level of infestation by C. multicicatrices compared to trees and palms and other growth habits (s.r. = 2.37; p < 0.05).
Several plants had a higher level of infestation than others (Table 2). These host plants are leguminous species Albizia pistaciifolia, Samanea saman, and Cassia siamea; fruit trees Mangifera indica, Psidium guajava, Citrus sinensis, C. reticulata, Annona muricata, and A. cherimola; and palms Phoenix roebelenii, Wodyetia bifurcata, and Roystonea oleracea. Kondo, Becerra et al. (2014) also reported on San Andrés Island (Colombia) that the highest level of infestation by C. multicicatrices occurred on palms, various fruit trees, and leguminous trees and shrubs.
We detected high levels of infestation by C. multicicatrices on Pradosia montana (Sapotaceae) and Erythrina smithiana (Fabaceae), both endemic to Ecuador; E. smithiana is categorized as “endangered” (Neill, 2017). Future studies are needed to monitor the potential impact of C. multicicatrices on E. smithiana populations.
Considerations for management
Urban green areas promote human well-being (Bertram & Rehdanz, 2015) and are necessary for mitigating the effects of climate change in cities (Nero et al., 2017). Crypticerya species can reduce the photosynthetic rate of infested plants and cause defoliation and death of the host (Kondo et al., 2012), significantly affecting vegetal cover in urban green areas. Management of the invasion by C. multicicatrices and C. genistae is necessary to avoid the loss of the benefits that urban green areas provide to citizens. Control of invasive species includes prevention, eradication, and containment.
Interrupting the dispersal pathway(s) is necessary to prevent new introductions of these invasive fluted scale species to non-infested areas. Kondo et al. (2012) suggested that the introduction of C. multicicatrices to San Andrés Island occurred through the transport of infested ornamental plants from continental Colombia. It is probable that trade in ornamental plants and ineffective pest control in local nurseries facilitated the introduction and spread of C. multicicatrices and C. genistae in Guayaquil, Samborondón, and Daule. Hence, it is essential to devise protocols that strengthen the control of these two species of fluted scales during trade in ornamental plants and the effectiveness of pest control on plants in local nurseries. Another prevention strategy is to plant a wide variety of trees and shrubs belonging to different plant families in urban green areas to include plants resistant to the fluted scales.
Biological control has been suggested as the most effective strategy for eradicating invasive Crypticerya species (Etienne & Matile-Ferrero, 2008; Gaimari et al., 2012; González et al., 2012; Kondo et al., 2012). In neighboring Colombia, many natural enemies of C. multicicatrices have been reported, including an entomopathogenic fungus, Isaria sp. (Fungi: Eurotiales: Trichocomaceae); predators including ladybird beetles, i.e., Delphastus quinculus Gordon, Diomus seminulus (Mulsant), Novius cardinalis (Mulsant), and N. punicus (Gordon) (Coleoptera: Coccinellidae); a predatory fly, Syneura cocciphila (Coquillet) (Diptera: Phoridae); two species of predatory lacewings, Chrysoperla sp., and Ceraeochrysa sp. (Neuroptera: Chrysopidae); a parasitoid wasp, Brethesiella cf. abnormicornis (Girault); and a hyperparasitoid wasp, Cheiloneurus sp. (Hymenoptera: Encyrtidae) (Kondo, Ramos-Portilla, et al., 2016). Many of these insects may also likely occur in Ecuador because of the two countries’ proximity.
Although many native host-plant species are found in the dry forests adjacent to Guayaquil, Samborondón, and Daule, the invasion of C. multicicatrices and C. genistae is restricted to urban green areas, implying the presence of natural enemies in the dry forest in the study area that could be used as biological control agents. Further studies are required to identify the natural enemies of both invasive species in this dry forest.
On the other hand, the use of pesticides to control invasive Crypticerya species has been reported as counterproductive in the mid- and long term because they reduce populations of the natural enemies (Kondo et al., 2012). Early detection and rapid response through systematic surveillance of urban green areas are required to prevent infestations by C. multicicatrices and C. genistae. Infestations by Crypticerya species are generally more frequent during the dry season (Kondo et al., 2012); thus, the surveillance of urban green areas should be more frequent and thorough during this season.