INTRODUCTION
Tripterygiidae is a family belonging to the order of Blenniformes that includes 32 genera and 183 valid species, 16 of which have been described during the last 10 years (Fricke et al., 2020). The so-called triplefins are found both in warm and tropical oceans around the world and they live associated with coral or rocky seabeds. Most of their species have small sizes (< 6 cm), feature that, along with their cryptic colors, make them hard to detect (Robertson y Allen, 2015; Nelson et al., 2016).
They are distinguished from other families by having the dorsal fin divided into three distinct segments. The first and second parts are made of spines, and the third one has more than seven soft rays. They lack cirri on the nape, but they have them in the nostrils and over the eyes; they usually have ctenoid scales, branchial membranes broadly joined to the isthmus, and protractile premaxillaries (Nelson et al., 2016). They have benthic eggs cared for by the male, joined to the nest site through adhesive filaments (Ruck, 1973, 1980; Shiogaki y Dotsu, 1973; Wirtz, 1978), spherical to slightly flattened, 0.7-1.4 mm in diameter, and with a colorless to red-orange yolk having 10 or more little oil drops. Larvae are 3-10 mm long, without scales on the head, pigmented in the ventral postanal rim and they have a little yolk sac (Ruck, 1973, 1980; Shiogaki and Dotsu, 1973, 1988, Beltrán-León and Ríos, 2000).
In the Neotropical region, from the southern region of Mexico to South America in the American Pacific (Sclater, 1858), four genus and 24 species are distributed, eight of them described in the current century (Rosenblatt et al., 2013; Victor, 2013). For this region, the most diverse genus is Enneanectes, with 15 species distributed between the tropical eastern Pacific (TEP) and the western Atlantic (Rosenblatt, 1960; Robertson y Allen, 2015). The remaining three genera are endemic to the TEP: Axoclinus (six species), Lepidonectes (three species), and Crocodilichthys (one species); five of these species are unique to the islands or archipelagoes comprising the TEP oceanic province (Clipperton, Revillagigedo, Galápagos, Coco Island, and Malpelo Island; Robertson and Cramer, 2009).
Malpelo Fauna and Flora Sanctuary (FFS) harbors two of the five endemic species to the oceanic province of the TEP: Axoclinus rubinoffiAllen and Robertson, 1992 and Lepidonectes bimaculatus Allen and Robertson, 1992, which live in depths shallower than 30 m and are associated to rocky sea beds covered by encrusting coralline algae (Chasqui-Velasco et al., 2011). Due to their reduced body length and low swimming capacity, it is assumed that they have a limited dispersion potential (Rocha y Bowen, 2008), at least during their young and adult stages. These two species represent the most abundant endemic fish in the island (Chasqui-Velasco et al., 2011), compared to the other three endemic species: the chaenopsid Acanthemblemaria stephensi Rosenblatt and McCosker, 1988, the goby Chriolepis lepidota Findley, 1975 and the Labrid Halichoeres malpelo Allen and Robertson, 1992.
The endemic species have registered high extinction rates (Frankham, 1997) due to their biological (limited dispersion), ecological (small populations, habitat specialists), and genetic (low genetic flux and low genetic diversity) characteristics (Pimm and Pimm, 1991; Ellstrand and Elam, 1993) that make them especially vulnerable to perturbations and place them in a higher risk of extinction than broadly distributed species (Hughes et al., 2002). Given this vulnerability, many studies have been conducted to understand and preserve their populations (Ellstrand and Elam, 1993; Hamrick and Godt, 1996). Despite the FFS Malpelo’s endemic are cataloged as vulnerable in the Colombia Red Book of Marine Fish (Zapata and Chasqui-Velasco, 2017a, 2017b) and by the International Union for the Conservation of Nature (IUCN) (Hastings et al., 2010a, 2010b), there is little available information about these species. Works carried out to date respond to the abundance, distribution, and ecology (Quimbayo et al., 2010; Chasqui-Velasco et al., 2011). The original description of the species provides the only available morphological data of the adult stage, limited to the few individuals collected for this purpose (Allen y Robertson, 1992). This work complements the information on morphology, abundance, and distribution of the adult stage of Axoclinus rubinoffi and Lepidonectes bimaculatus, and reports the first records and descriptions of larval stages for these two endemic species of the FFS Malpelo.
STUDY AREA
The oceanic island Malpelo is located in the TEP and is approximately 500 km away from the Colombian coast. It is the peak of the Malpelo dorsal, a solitary submarine volcanic ridge, which probably emerged 16-17 million years ago (Hoernle et al., 2002); it has not been connected to the continental platform or any other island, not even in shallow waters (Chase, 1968; Lonsdale and Klitgord, 1978). Malpelo Island has sheer walls and small platforms with underdeveloped coral formations (Graham, 1975). Due to its geomorphological features and to a high diversity of marine species, Malpelo Island together with its surrounding waters was designated a Fauna and Flora Sanctuary since 1995 (Rodríguez-Rubio and Giraldo, 2011). The Malpelo FFS is one of the 59 protected areas of the Colombia National System of National Natural Parks, was recognized as Natural Heritage by UNESCO in 2006 and recently joined the IUCN’s Green List of Protected and Conserved Areas.
MATERIALS AND METHODS
A total of 11 adult individuals of every species were captured around the island between July 4th and November 20th, 2018, including the islets found to the north and the south (Figure 1). The collection was carried out using hand nets, and individuals were taken to a glass aquarium where they were numbed with a lethal dose of Tricaine Metasulfonate approved by the Food and Drugs Administration for fish handling (Carter et al., 2011). Organisms were fixed in 10 % ethanol and they were deposited in the Ichthyologic Collection of the Universidad del Valle (CIR-UV) under the catalog numbers 18134-18146 for L. bimaculatus and 18148-18154 for A. rubinoffi.
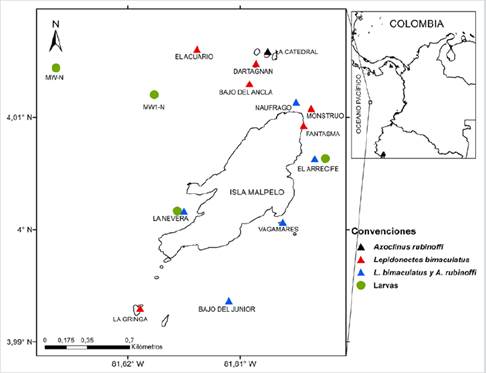
Figure 1 Distribution of Axoclinus rubinoffi and Lepidonectes bimaculatus larvae and adults collected during 2015 and 2017-2019 in FFS Malpelo. Triangles correspond to organisms captured in the adult stage and circles to larval stages.
The measurements, counts, and descriptions were made following Allen and Robertson (1992). Measurements of total length (TL), standard length (SL), body height (BH), body width (BW), head length (HL), snout length (SNL), eye diameter (ED), interorbital distance (ID), caudal peduncle height (CPH), caudal peduncle length (CPL), and pectoral fins length (PFL) were considered. The measurements were made using a 0.1 mm precision caliper. Also, counts regarding the elements (spines and rays) of the dorsal (DF), anal (AF), pectoral (PF), pelvic (PVF), and caudal fins (CF) were made.
The density (individuals/m2) of each species were calculated using the data from a monitoring in July 2018 carried out by Ecology of Coral Reefs research group (Universidad del Valle). The monitoring was carried out in four locations in the island: La Nevera, El Arrecife, La Pared del Náufrago, and El Bajo de Junior. The protocol designed by Chasqui-Velasco et al. (2011) was followed, which consists of visual census using scuba diving equipment along 20 m x 2 m belt transects, which correspond to 40 m2 and allows making a standardization to the number of individuals per square meter.
Individuals in early-stage (larvae) were collected in March 2015 in stations MW-N and MW1-N (schedule established by Project INPA - DIMAR 1993), located on the west side at 0.8 and 1.61 km from Malpelo. During September 2017 and May, 2019 individuals were also captured in locations Arrecife and La Nevera, where superficial dragging was carried out very close to the island (Figure 1). Samples were obtained by dragging with bongo nets of 300 and 500 µm meshes, soft collecting flakes, and analogous flow counters. The dragging was oblique until a maxumim depth of 50 m in stations MW-N and MW1-N, and superficial in stations El Arrecife and La Nevera, following the methodology by Smith and Richardson (1979). The samples were fixated with 10 % formaldehyde neutralized with borax in seawater. The larval description was made with four individuals of A.rubinoffi and two of L. bimaculatus.
The identification of de A. rubinoffi and L. bimaculatus larvae was carried out based on the meristic, morphometric, and pigmentation characters, following the identification keys of Tripterygiidae family by Moser (1996) and Beltrán-León And Ríos (2000). In all cases, the number of organisms was standardized to individuals/m2. Finally, the larval stages were photographed in the Images Laboratory of the Universidad del Valle’s postgraduate program in Science-Biology, using a NIKON MNA43000 stereoscope and a NIKON MQA16050 camera.
RESULTS
Rubinoff’s triplefin
Axoclinus rubinoffi Allen and Robertson, 1992
Morphology.- The only larvae found in the flexion stage had 6.2 mm total length, 5.3 mm standard length, elongated body; moderate preanal length (LpA) 43 % SL; HL (20-33 % SL) and ED (25-33 % HL); during this stage, they have the third fin (soft rays) made of 10 rays, anal fin with II bones and 17 soft rays. During this stage begins the formation of the two first dorsal (spines) and pelvic fins, until completion of the development of all fins; with 39 myomeres.
In the adult stage, the body is short and robust; head with cirri in the nostrils and over the eyes; three dorsal fins III-XII-10; anal fin II-17; pectoral fins with 15 rays, rarely 14; pelvic fin I-2; a lateral line descends from the superior edge of the operculum to the middle lateral axis; five scales over the lateral line to the base of the first caudal ray; four scales below the lateral line to the base of the anal rays; convex caudal fin with 13 rays. Morphometric and meristic data are shown in detail in Table 1.
Table 1 Morphometric and meristic characters of Axoclinus rubinoffi y de Lepidonectes bimaculatus benthic individuals.

Total length (TL), standard length (SL), body height (BH), body width (BW), head length (HL), snout length (SNL), eye diameter (ED), interorbital distance (ID), caudal peduncle height (CPH) and pectoral fins length (PFL). Rays and spines count of dorsal (DF), anal (AF), pectoral (PF), and pelvic (PVF), and caudal (CF) fins. Measurements are shown with respect to SL or HL, except BW, which is calculated in relation to BH and ID with respect to ED.
Coloration.- Larvae are transparent, and in the flexion stage they show chromatophores in the head over the skull and the cleithral symphysis, in the body close to the anus, in ventral post-anal series (17), a stronger one between the series and the caudal peduncle, three above the caudal peduncle and the hypural joint; internal pigments in the nape and the anterior intestine (Figure 2 A, B, C, and D). Adults distinguish by the white ventral surface of the head and their whitish body; four wide brown bars between the base of the pectoral and caudal fins, the two posterior ones more conspicuous than the two anterior ones, all the bars with white edges, pale brown color between them; a short bar with white edges behind the inferior end of the eye; translucent dorsal, anal, and pectoral fins, pectoral fin with a white stain on its base, white first rays; there are differences in the coloration of the caudal fin between sexes, males have them black, females fin with orange coloration (Figure 2 E, F, G, and H).

Figure 2 Photographs of Axoclinus rubinoffi in Malpelo Island, Colombia. A) Larval stage, side view A). B) Larval stage, dorsal view. C) Larval stage, ventral view. D) Close up, side view. E and H) Adults on encrusting coralline algae. F) Coloring of two sexes, male (inferior). G) Adult on filamentous algae. Credits of photographs A, B, C and D: © Images Laboratory of the Universidad del Valle’s postgraduate program in Biology-Ortega, Beltrán- León; E: ©Paola María Sánchez; G: ©B Guenard; H: ©Andrés Felipe Acosta.
Habitat.- Larvae are planktonic and they are mainly found in shallow waters close to the rocky area. They were captured at night during March 2015, in MW-N and MW1-N (Figure 1), to the west of Malpelo, with 24.9 °C of surface temperature; superficial salinity: 30.62-30.66 (mean = 30.64); sampling depth: 27.9-29.3 m (mean = 8.6 m); distance from the island: 0.8 and 1.61 km; time: 21:12 - 21:55. During September 2017 they were captured at night in El Arrecife and La Nevera. Surface Temperature: 26.1-26.2 °C (mean = 26.15 °C); superficial salinity: 31.2-31.3 (mean = 31.25); sampling depth: 1 m; distance from the island: 20 m approximately; time: 19:34 - 20:13. Captured in May 2019 in La Nevera to the west of the island at night, superficial temperature: 28.05 °C; superficial salinity: 32.98; sampling depth: 1 m; distance from the island: 20 m approximately; time: 18:35. On the contrary, adults are benthonic inhabitants of rocky seabed covered with encrusting coralline algae and filamentous algae (Figure 2E, G, and H). They were collected between 0-30 m depth, during daytime and in the locations shown in figure 1.
Abundance.- Larvae density was low in the stations where they were captured: 5.8 individuals/m2 in MW-N and 2.7 individuals/m2 in MW1-N during March 2015. Tripterygiidae family showed a relative abundance of 1.03 % of the total of collected larvae. In El Arrecife it was 0.1 individuals/m2 and 0.3 individuals/m2 in La Nevera, with a relative abundance of 0.70 % during September 2017. In May 2019, 0.5 individuals/m2 in La Nevera, with a relative abundance of 0.41 % of the total of collected larvae.
A. rubinoffi (adults + young) density in El Arrecife (640 m2 sampled) was 0.02 individuals/m2; in La Nevera (600 m2 sampled) 0.07 individuals /m2, in La Pared del Náufrago (600 m2 sampled) 0.10 individuals/m2 and in El Bajo del Junior (560 m2 sampled) 0.56 individuals/m2. The species average density was 0.19 individuals/m2 for the four locations sampled (2,400 m2).
Conservation status.- Vulnerable D2 in the UICN’s Red List of Endangered Species, and Colombia’s Red Book of Marine Fish (Hastings et al. 2010a; Zapata and Chasqui-Velasco, 2017a).
Similar Species.- Tripterygiidae larvae resemble Labrisomidae but they generally distinguish by combinations of pigmentation, meristic and morphometric characters. The three dorsal fins of this species’ family clearly distinguish them from other Blennioidei larvae once they reach 10 mm TL. Axoclinus rubinoffi (D III+XII+10; A II,17-18; Pc 15) is endemic to Malpelo and can be distinguished from other similar species by meristic: Lepidonectes bimaculatus (D III+XIII,10-11; A II,19; Pc 17) and A. lucillae (D III+XII+9; A II, 17; Pc 15-16); the latter is not distributed in the island, but it is found in shallow water along the TEP coast. In the adult stage, A. cocosensis Bussing (1991), endemic to Coco Island, shows similar morphology and coloring; however, A. rubinoffi differs from this species because it has no white line separating the peduncle bar and the caudal fins.
Twin-spot triple fin
Lepidonectes bimaculatusAllen and Robertson, 1992
Morphology.- The only larvae found in the flexion stage had 8.1 mm total length; 7.1 mm standard length; elongated body; LpA 45 % of SL; HL (20-33 % of SL) and eye (ED 25-33 % of HL) moderate; during this stage they have the third fin formed by 11 rays, anal fin with II spines and 20 rays; formation of the two first dorsal fins (hard rays or spines) and the pelvic ones starts, until completing the development of all fins; with 43 myomeres.
The adult stage showed a slightly elongated body; big cirrus over the eye; spinules on the head and on the base of the first dorsal ray; three dorsal fins III-XII-11, rarely 10; anal fin II-19, rarely 18; pectoral fins with 16 rays; lateral line in two sections; two scales over the lateral line to the base of the first caudal ray; six scales under the lateral line to the base of the anal ray; pelvic fin I-2 and convex caudal fin with 14 rays. Morphometric and meristic data are shown in detail in Table 1.
Coloration.- Larvae are translucent and in the flexion stage they have no pigment on the head. They have pigment close to the cleithral symphysis, in the body close to the anus, 22 chromatophores in postanal ventral series, three and one over the anal peduncle, a small one in the center of the caudal peduncle, and internal pigments in the anterior part of the intestine and over the swim bladder (Figure 3 A, B, C, and D). Young ones have the superior half of the head with grey to dark blue color and irregular iridescent lines, the inferior half of the head is whitish; body with dark brown bars that form a wide half-lateral strip; thin bars with reddish coloration in the superior half of the body and white inferior half; two white dots on the upper half of the body; white first dorsal fin; translucent second and third dorsal fins; translucent caudal fin; yellow pectoral and pelvic fins; black dot in the caudal peduncle (Figure 3 E, and F). Adults have an orange coloration in the superior half of the body; inferior half with yellow color; white edge in the anal and caudal fins; two white saddles on the posterior half of the body; females with orange coloring in the base of the dorsal fin and whitish exterior margin; males with yellowish dorsal fin base, black rays and black exterior margins; yellow pectoral and pelvic fins; brown blotch in the caudal peduncle, more evident in males (Figure 3G, and H).

Figure 3 Photographs of Lepidonectes bimaculatus in Malpelo Island, Colombia. A) Side view of larval stage. B) Dorsal view of larval stage. C) Ventral view of larval stage. D) Side view close-up of larval stage. E and F) Young ones. G) Photograph of a female. H) Photograph of a male and a female. Photograph credits A, B, C and D: © Images Laboratory of the Universidad del Valle’s postgraduate program in Biology-Ortega, Beltrán-León; E, F, G: ©Paola María Sánchez.
Habitat.- Larvae are planktonic and they were mainly found in shallow waters on the rocky area, habitat of adults. They were captured in El Arrecife to the east of the island and La Nevera to the west of the island at night during September 2017. Superficial temperature: 26.1-26.2 °C (mean = 26.15 °C); superficial salinity: 31.2-31.3 (mean = 31.25); sampling depth: 1 m; distance from the island: 20 m approximately; time: 19:34-20:13. Captured in May 2019 in La Nevera to the west of the island at night, superficial temperature: 28.05 °C; superficial salinity: 32.98; sampling depth: 1 m; distance from the island: 20 m approximately; time: 18:35. Adults are benthonic inhabitants of rocky seabed covered with encrusting coralline algae (Figure 3E, F, G, and H). They were collected between 0-30 m depth, during daytime and in locations shown in figure 1. It is usual to find the young ones on exposed rocks, while adults stay more time in crevices and the internal face of rocks, usually in an inverted position (Figure 3F, G, and H).
Abundance.- Larvae density was low in the stations where individuals were captured. In El Arrecife, it was 0.2 individuals/m2, and in La Nevera 0.3 individuals/m2. Tripterygiidae family showed a relative abundance of 0.87 % of the total of collected larvae during September 2017.
Regarding adults and young ones, L. bimaculatus density in La Nevera (600 m2 sampled) was 0.12 individuals/m2, in El Arrecife (640 m2 sampled) 0.15 individuals/m2; in El Bajo del Junior (560 m2 sampled) 0.17 individuals/m2 and in La Pared del Náufrago (600 m2 sampled) 0.45 individuals/m2. For the four locations sampled (2,400 m2) the average density of the species was 0.22 individuals/m2.
Conservation status.- Vulnerable D2 in the UICN’s Red List of Endangered Species, and Colombia’s Red Book of Marine Fish (Hastings et al. 2010a; Zapata and Chasqui-Velasco, 2017a).
Similar Species.- Lepidonectes bimaculatus larvae (D III+XIII, 10-11 A II, 19 Pc 17) can be distinguished from other similar species in the area by meristic: A. rubinoffi (D III+XII+10 A II, 17-18 Pc 15) and A. lucillae (D III+XII+9 A II, 17 Pc 15-16). Lepidonectes bimaculatus shows similar shape and counts to L. clarkhubbsi Bussing, 1991; however, they differ in their coloration, because the L. clarkhubbsi young ones have distinctive bars in the lateral area of the body and females have whitish coloring.
DISCUSSION
This work provides morphological data of larval stages of the endemic species A. rubinoffi y L. bimaculatus in the Malpelo Flora and Fauna Sanctuary. It also provides information that complements the abundance, distribution, and morphology of the adult stage, adding measurements of a series of individuals greater than those employed in the original description made by Allen and Robertson (1992), which allows a better understanding of the morphologic and meristic variability of these endemic species.
Although 27 scientific expeditions since 2006 have taken place to monitor ichthyoplankton in FFS Malpelo in different seasons of the year, in only one (3.7 %) of the expeditions conducted (March 2015) larvae of the Tripterygiidae family were captured, corresponding to two of the six traditionally sampled stations. Besides, these species were captured in two (50 %) of the expeditions where new stations were involved on El Arrecife and La Nevera. This could indicate that larvae of this family are commonly found in places close to hard substrates, mainly where adults live in.
It is highlighted that adults of the species A. rubinoffi were collected in seven sites, two of which the species was found in the larval stage. In the case of L. bimaculatus, it was collected in 11 sites, finding its larvae in two of them. Due to the presence of adults in all the collecting sites, it is suggested that reproduction and therefore larvae, must be present in all the island sites where the adults are distributed, but given the lack of plankton draggings close to the island and associated to the reef and hard substrates, they have not been collected before. Doing this sampling would give the opportunity of obtaining more stages that allow describing their entire ontogenic development, as only larvae in the flexion stage were captured. Despite the few larvae captured, their development stage indicates that little time has elapsed since their hatching, so it could be speculated that adults have reproductive peaks during March, May, and September.
The number of examined individuals in this research allows enlarging the current knowledge on the natural variability of these two species regarding their morphologic and meristic features. Counts of elements in A. rubinoffi adults show similar values in the dorsal, anal, pelvic, and caudal fins, but some differences in the counts of pectoral rays with respect to the original description by Robertson y Allen (1992). L. bimaculatus also shows similar counts in the dorsal, pelvic, and caudal fins, with slight differences in the number of rays in the anal and pectoral fins. Differences found in the morphometry were expected because of the greater number of individuals measured in this work, including a greater size range for both species (14.8-24.5 mm SL in A. rubinoffi and 24.9-47.7 mm SL in L. bimaculatus).
The average density of A. rubinoffi (0.19 individuals/m2) and the values obtained for El Arrecife (0.02 individuals/m2) are similar to those found almost a decade ago by Chasqui-Velasco et al., 2011 (0.18 individuals/m2 and 0.024 individuals/m2 respectively). In contrast, densities of about a half are recorded for El Bajo de Junior (0.56 individuals/m2), La Nevera (0.07 individuals/m2) and La Pared del Náufrago (0.10 individuals/m2). Regarding L. bimaculatus, similar values were observed in both works for La Nevera. Chasqui-Velasco et al. (2011) report lower values in Bajo del Junior (0.09 individuals/m2), La Pared del Náufrago (0.084 individuals/m2) and El Arrecife (0.043 individuals/m2 ), as well as for the average density of the species (0.08 individuals/m2 ). The values obtained in this work for the same locations were (0.17 individuals/m2; 0.45 individuals/m2; 0.15 individuals/m2 and 0.22 individuals/m2, respectively).
The differences found between densities in both works could be due to the sampling effort made in each of them and to the demographic variations inherent to species during the different monitoring seasons. Studies with a larger time series and other methodologies such as genetic evaluations of the effective population size are required. Such indicators have been deemed important because they allow making inferences on the persistence of the endemic species in the long term (Ellstrand and Elam, 1993), due to processes such as genetic drift, endogamy, bottlenecks, and founding events affect their diversity and differentiation (Lammi et al., 1999). Besides, they have shown sometimes information that is not consistent with field data, for instance, the effective population size can be lower than the census population sizes (Turner et al., 2002). It is important that this information could be compared to genetic indicators to better understand the dynamics of the species and to take more efficient conservation and handling actions.
CONCLUSIONS
Larvae of the species A. rubinoffi y L. bimaculatus were found in the water column over the adults’ habitat, hard substrates such as rocky or coralline seabeds, confirming the importance of maintaining samplings in the new stations, which allow capturing species associated with the reef that hardly could be captured in the rest of the sampled stations.
The initial stage of larval development found suggests that adults have reproductive peaks during March, May, and September.
This study provides information to understand vulnerable species defined as objects of conservation for FFS Malpelo. Knowledge of the natural variability of the morphologic ranges is enlarged and data for the understanding of biological factors that drive the success of endemic species are provided.
The average density of A. rubinoffi is similar to that found by other researchers in 2011. On the contrary, differences are detected between population densities for L. bimaculatus, showing higher densities than in 2011. To understand the population dynamics of these species it is necessary to assess time series, implement other methodologies such as genetic evaluations, and keeping their monitoring and investigation.











 text in
text in 



