INTRODUCTION
Ichthyoplankton are an important component of marine zooplankton and the factors that influence its distribution and abundance are the same as those affecting other plankton: food requirements (adequate dietary availability at appropriate times and in sufficient quantities), the presence of predators (including methods to avoid predation) and the influence of temperature, salinity, currents and other physical and chemical factors (Giraldo et al., 2014). Their ecological importance lies in the fact that, as structural and functional components of aquatic ecosystems, variability in their composition and abundance illustrates their quality of their surroundings or its deterioration (Beltrán-León and Ríos, 2000). The marine protected areas of the Gorgona and Sanquianga Natural National Parks (NNPs) and the Malpelo Fauna and Flora Sanctuary (FFS) play a very important role in the dispersal and recruitment of larvae, which are key to the maintenance and restocking of fishery resources in surrounding waters, as has been observed for islands in other parts of the world (Swearer et al., 1999). Increased exploitation of coastal and oceanic waters of the Colombian Pacific has increased pressures on fishing grounds and led to increased interest in extending this exploitation to protected areas. Approaches to sustainable development must therefore be adjusted in response to these challenges, and decisions taken and implemented that incorporate conservation, the sustainable use of resources and the equitable share of benefits of development, without affecting the fundamental conditions required for the continuation of life (Beltrán-León and Ríos, 2000). Consequently, studies of ichthyoplankton are important for evaluating fish stocks in a given area, making it possible to establish information on the composition, diversity and productive potential of a zone while simultaneously identifying the areas and periods that are critical to the development of fisheries resources (Beltrán-León and Ríos, 2000). In November 2009 the Dirección Territorial Suroccidente (Southwest Territorial Directorate) now known as the Dirección Territorial Pacífico (Pacific Territorial Directorate ), part of the Sistema de Parques Nacionales Naturales (System of Natural National Parks), carried out a series of scientific expeditions to the Sanquianga and Gorgona NNPs and the Malpelo FFS, in order to gather information on fish eggs and larvae, determine spawning areas and nurseries and establish the role and influence of the environment on the early stages of life. The scientific expeditions also produced an inventory of biodiversity, that, in conjunction with periodic monitoring, will make it possible for future decision-making to incorporate conservation practices and the sustainable use of resources.
STUDY AREA
The protected areas of the Gorgona NNP (02° 58′ 17″ N - 78° 11′ 04″ W), the Sanquianga NNP (2° 38′ 59″ - 78° 17′ 09″ W) and the Malpelo FFS (Point 1: 4° 26’ 00″ N - 82° 00’ 00″ W, Point 2: 4° 26’ 00″ N - 81° 08’ 00″ W, Point 3: 3° 32’ 00″ N- 82’ 00’ 00″ W, Point 4: 3° 32’ 00″ N - 81° 08’ 00″ W), are among the 59 protected areas covered by the Natural National Parks System. They are located in the coastal and oceanic zone of the Colombian Pacific (Figure 1).
MATERIALS AND METHODS
The sampling plan consisted of 24 stations located in the marine area offshore from the Sanquianga NNP, using a grid established by the 2008 joint WWF-Colombia/Ministry of Agriculture Small Pelagics Project (Small Ocean Projects) (Zapata et al., 2011), a further 24 stations in the Gorgona NNP, using a grid established by the 2006 Univalle - Colciencias project (Escarria et al., 2007), and 12 stations off the coast of the Malpelo FFS, using a grid established by the 1993 INPA - DIMAR project (Beltrán-León, 2007). All samples were taken during November 2009 (Figure 2).

Figure 2 Location of the sampling stations A. Gorgona NNP, B. Sanquianga NNP and C. Malpelo FFS, Colombian Pacific.
Surface trawls for zooplankton were conducted in the Sanquianga NNP (24 stations) and the Malpelo FFS (12 stations), while for logistical reasons and because of the nature of the sample design, oblique trawls were conducted in the Gorgona NNP (24 stations) to a maximum depth of 50 m, using the methodology established by Smith and Richardson (1979). The samples were collected using bongo nets of 300 and 500 µm and Hydrobios analogue flowmeter. Samples were preserved using formalin buffered at 10 % in sea water. All the trawls were carried out at a speed of 3.7 km/h. Simultaneously, surface temperature and salinity were measured for all stations using a YSI portable system to measure conductivity, salinity and temperature; water column transparency was recorded using a Secchi disk. Once the samples had been transferred from the sampling zones to the laboratory in the city of Cali, the biomass of the zooplankton was determined using the volumetric method and the fish eggs and larvae (ichthyoplankton) were separated from the rest of the zooplankton (planktonic invertebrates), which had been collected using the 300 µm net.
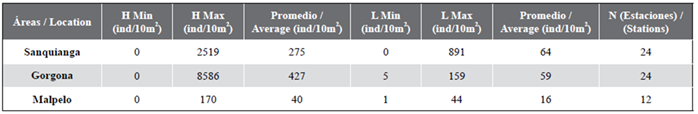
The fish larvae were identified by their meristic and morphometric characteristics and pigmentation to the lowest taxonomic level possible (family, genus and/or species), using the identification criteria developed by Moser (1996) and Beltrán-León and Ríos (2000). In all cases the number of organisms was standardized to individuals/10 m². The salinity, temperature and abundance ranges recorded by Rueda-Montenegro and Beltrán-León (1992), and modified by Beltrán-León et al. (1994), were used for reference (Table 1). A 5 % significance level of was employed in all the analyses. The correlations between the two physico-chemical variables (temperature and salinity) and the five biological ones (zooplanktonic biovolume, fish eggs, fish larvae and Engraulidae eggs and larvae) were examined using the Pearson correlation coefficient. The following steps were adhered to in order to compare the seven variables (two physico-chemical and five biological) in the three protected areas:
Normality test for each of the three subsets of data, using the Lilliefors test (Kolmogorov-Smirnov).
Homoscedasticity test between the three subsets of data, using the Fligner-Killeen test.
Means equality test (or averages depending on the case): i. If normality and homoscedasticity were found, using a standard ANOVA. ii. In the event of normality and homoscedasticity, using a Welch ANOVA. iii. If there was no normality, the distributions were similar and therefore homoscedasticity was found, using a non-parametric Kruskal-Wallis ANOVA. iv. If there was no normality, the distributions were similar and there was heteroscedasticity, using a non-parametric Bootstrap ANOVA (Hesterberg, 2015).
The degree of association between the abundance of fish larvae and physico-chemical parameters was evaluated using a non-parametric correlation of an analysis with nMDS (non-Metric Multidimensional Scaling) and the environmental matrix. All the information obtained from the sample and the laboratory analyses were standardized to individuals /10m2 in order to render the data comparable.
RESULTS
Oceanographic conditions
Sea surface temperature (SST) during November 2009 oscillated between 26.5 and 29.2 °C. The lowest temperature was recorded in Gorgona, rising by 0.2 °C in Sanquianga and by 0.9 °C in Malpelo. The SST data for Sanquianga and Malpelo is considered normally distributed (p = 0.596 and 0.138), but this was not the case for Gorgona (p = 0.015), which was statistically different (p < 0.001). The temperatures around Malpelo are higher than those of Sanquianga and Gorgona (p < 0.001), but Sanquianga and Gorgona do not differ between themselves (p = 0.691) (Figure 3, Table 2).
Table 2 Sea Surface Temperature (SST °C) and Sea Surface Salinity and (SSS) in the Sanquianga and Gorgona NNPs and Malpelo FFS, Colombian Pacific, November 2009.

Sea surface salinity (SSS) oscillated between 2.9 and 35, with average salinity lowest in Sanquianga, increasing by 10.9 in Gorgona and by 13.2 in Malpelo. The salinity data for Sanquianga and Gorgona is considered normally distributed (p = 0.073 and 0.056), but not for Malpelo (p = 0.007). Sea surface salinities differed statistically between the three areas (p < 0.001), levels being highest in Malpelo, followed by Gorgona and Sanquianga (p < 0.003). (Figure 3, Table 2).
Zooplankton biomass
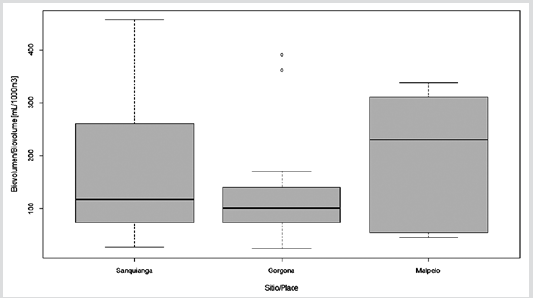
Zooplankton biovolume oscillated between 24.7 and 455.6 ml/1000 m3. The biovolume for Malpelo was normally distributed (p = 0.115), but this was not the case for Sanquianga and Gorgona (p = 0.006 and 0.009). The biovolumes did not differ statistically between the three areas (p = 0.089), lying within the average biomass range in all three areas (Figure 4. Table 3).
Table 3 Biovolume of zooplankton in the Sanquianga and Gorgona NNPs and the Malpelo FFS, Colombian Pacific, November 2009.

Fish eggs
A total of 17,341 eggs/10 m2 were collected, of which 6,603 eggs/10 m2 came from the Sanquianga NNP, 10,256 eggs/10 m2 from the Gorgona NNP and 482 eggs/10 m2 from the Malpelo FFS. Fish egg numbers were not normally distributed in any of the three protected areas (p < 0.009) and the homoscedasticity assumption was not rejected (p = 0.063). No significant differences were found between the three areas (p = 0.73). Values were highest in Gorgona, followed by Sanquianga and Malpelo (Figure 5, Table 4). Low abundance levels were reported for fish eggs in 62.50 % of the stations in Sanquianga, 70.83 % of those in Gorgona and 85.00 % in Malpelo.
Total abundance of eggs from the family Engraulidae was 9,312 eggs/10 m2, of which the greatest abundance (8,586 eggs/10 m2) was found at station 17, northeast of the Gorgona NNP. The number of eggs per station were not normally distributed in any of the three areas, and the homoscedasticity assumption was rejected (p < 0.001). The differences in the Engraulidae egg count between the three areas were significant (P = 0.001), the greatest abundance being found in Gorgona, followed by Sanquianga and, lastly, Malpelo.
Table 4 Abundance of eggs (H) and fish larvae (L) in the Sanquianga and Gorgona NNPs and the Malpelo FFS, Colombian Pacific, November 2009.
Fish larvae
A total of 3,155 larvae/10 m2 were collected, of which 1,553 larvae/10 m2 (49.22 %) came from the Sanquianga NNP, 1,411 larvae/10 m2 (44.72 %) from the Gorgona NNP and 191 larvae/10 m2 (6.05 %) from the Malpelo FFS. The distribution of fish larvae in Gorgona and Malpelo may be considered normal (p = 0.045 and 0.093), but not in Sanquianga (p < 0.001). Significant differences were found between the two areas in terms of homoscedasticity (p = 0.001). Larvae were more abundant in Gorgona than in Sanquianga and Malpelo (p = 0.008 and 0.003), but there were no significant differences between Sanquianga and Malpelo (p = 0.512) (Figure 5, Table 4). Overall, 9.17 % of the stations in Sanquianga, 79 % in Gorgona and 95 % in Malpelo presented low fish larvae abundance.
Larvae of Engraulidae were not found in Malpelo. According to the QQ plot graph and Shapiro-Wilk test (p < 0.001), the distribution of the number of larvae was not normal in either Gorgona or Sanquianga. There were significant differences in the numbers of Engraulidae larvae found in Sanquianga and Gorgona (p = 0.022). On average, more larvae were found in Sanquianga than in Gorgona.
Composition of the catch
A total of 42 families, 65 genera and 90 species were found in the three protected areas in November 2009, of which 16 families (38.10 %), 21 genera (32.31 %) and 32 species (35.56 %) were found in Sanquianga; 29 families (69.05 %), 38 genera (58.46 %) and 52 species (57.78 %) in Gorgona; and 12 families (28.57 %), 19 genera (29.23 %) and 21 species (23.33 %) in Malpelo (Table 5). The most abundant families found in the Sanquianga NNP were Engraulidae (anchovies) (64.39 %), Sciaenidae (croakers) (13.39), Gobiidae (gobies) (9.53 %), Haemulidae (grunts) (6.12 %) and 12 further families that made up the remaining 6.37 %. 0.19 % of the samples consisted of un-classified yolk-sac larvae (Table 5, Figure 6).
Table 5 Composition and relative abundance (%) of fish larvae present in the Sanquianga and Gorgona NNPs and Malpelo FFS, Colombian Pacific, November 2009.

For the Gorgona NNP the samples consisted of the families Bregmacerotidae (codlets) (17.65 %), Gobiidae (gobies) (13.11 %), Sciaenidae (croakers) (10.28 %), Haemulidae (grunts) (9.85 %), Trichiuridae (cutlass fish) (5.95 %), Paralichthyidae (flounders) (5.74 %), Engraulidae (anchovies) (3.97 %), Cynoglossidae (tonguefishes) (3.05 %), Serranidae (seabasses) (2,98 %) and 20 further families that accounted for 19.92 % of the total abundance. 4.89 % consisted of unclassified yolk-sac larvae and 2.62 % was classified as others (Table 5, Figure7). For the Malpelo FFS the catch consisted of the families Phosichthyidae (lightfish) (48.69 %), Gobiidae (gobies) (13.11 %), Myctophidae (lanternfish) (25.65 %), Hemiramphidae (halfbeaks) (11.52 %), Pomacentridae (damselfish) (3.66 %) and eight further families that accounted for 8.90 % of the total abundance. Others accounted for 1.57 % (Table 5, Figure 8).
Of the 42 families identified, 14.29 % (Engraulidae, Serranidae, Carangidae, Haemulidae, Sciaenidae and Gobiidae) were present both in Sanquianga and in Gorgona, 11.90 % (Paralepididae, Coryphaenidae, Pomacentridae, Paralichthyidae and Tetraodontidae) in Gorgona and Malpelo, 4.76 % (Phosichthyidae and Hemiramphidae) in Sanquianga and Malpelo and 2.38 % (Myctophidae) in all three protected areas. While 16.67 %, of the total -the families Pristigasteridae, Clupeidae, Belonidae, Gobiesocidae, Eleotridae, Scombridae and Achiridae- were found exclusively in the Sanquianga NNP, 40.48 % (the families Aulopidae, Synodontidae, Ophidiidae, Bythitidae, Bregmacerotidae, Merlucciidae, Mugilidae, Melamphaidae, Scorpaenidae, Gerreidae, Mullidae, Labridae, Tripterygiidae, Blenniidae, Microdesmidae, Trichiuridae and Cynoglossidae) were found exclusively in the Gorgona NNP; and 9.52 % -the Gonostomatidae, Exocoetidae, Lutjanidae and Nomeidae- were exclusive to the Malpelo FFS (Table 5).
The ordination analysis was very consistent, differences (evidenced by distance from the points) were observed in the richness and composition of species by separating the area into three groups: the Sanquianga NNP located in the coastal area (associated with axis 1, differentiated from Malpelo by salinity and temperature and from Gorgona by salinity), Gorgona in the intermediate zone (also associated with axis 1 and differentiated from Malpelo by temperature and from Sanquianga by salinity) and Malpelo, in the oceanic zone (associated with axis 2, differentiated from Sanquianga by salinity and temperature and from Gorgona by temperature). The clear differentiation between the distribution of the points in the localities by area is evident, a finding that supports the decision to separate the areas into the costal, intermediate and oceanic zones (Figure 9).

Figure 9 NMS analysis of site 1 red (Malpelo FFS), site 2 green (Gorgona NNP) and site 3 blue (Sanquianga NNP), November 2009.
Sanquianga NNP, located in the coastal zone, had the greatest species abundance, dominated by Cetengraulis mysticetus, with 935 larvae/10 m2 (60.21 %). The Gorgona NNP had lower abundance levels and was dominated by Bregmaceros bathymaster, with 249 larvae/10 m2 (17.65 %). The Malpelo FFS, in the oceanic zone, displayed very low fish larvae abundance compared to the other areas, with the species Vinciguerria lucetia predominating, with 93 larvae/10 m2 (48.69 %). In November 2009, Engraulidae eggs were found in the Gorgona NNP, a finding that might be normal. However, it did not have a high abundance. Similarly, it is surprising that eggs of this family were found in the surroundings of Malpelo, at night to the west and by day to the south.
DISCUSSION
Sea surface temperature at Gorgona and Sanquianga in November 2009 fell within the average ranges. These values are characteristic of warm waters. Average salinity at Sanquianga was low, which is to be expected given its position in the neritic zone and because it is influenced by large quantities of fresh water from the Patía and Sanquianga rivers.
For their part, salinity in the areas sampled in the Gorgona NNP and the Malpelo FFS were in the medium to high ranges, a result that is also to be expected in these areas and at the time of year, as both protected areas are far away from the coast and less prone to fluvial influence. This corresponds to the normal conditions found in these areas and season (May to December), characterized by low surface salinity values and a marked thermocline (at 40 to 50 m) (Stevenson et al. 1970; Giraldo, 2008; Giraldo et al., 2008a). For its part, the temperature in the Malpelo FFS was in the high range, coinciding with temperatures recorded for 2009 that were associated with a possible El Niño event (BAC, 2009). Events of this kind have significant climatic, oceanographic, biological and economic consequences for the entire planet (Chávez, 1987; Mann and Lazier, 1996; Levinton, 2001; Franco-Gordo et al., 2004), negatively affecting primary production and reducing the composition of the zooplanktonic biomass, which in turn affects the survival, reproduction and distribution of organisms at higher trophic levels, such as fish, birds and mammals (Chávez, 1996; Martínez-Aguilar et al., 2010).
In November 2009, biomass values were relatively stable, within average ranges for the month in the three areas sampled, providing evidence of significant secondary production that was apparently not affected by the high temperatures recorded for that month. November 2009 was not a significant spawning month and yolk-sac larvae were scarce, given that between 60 % and 95 % of the stations had low levels of abundance of eggs and larvae in Sanquianga, Gorgona and Malpelo. This might also be partially explained by the fact that the El Niño event is likely to have affected both the recruitment of larvae and the natural mortality rate of ichthyoplankton (Connol and Roughgarden, 1999; Hopcroft et al., 2002; Lavaniegos et al., 2002). During November 2009, the Sanquianga NNP had the greatest relative abundance, followed by the Gorgona NNP and the Malpelo FFS, while the Gorgona NNP was richest in species, followed by the Sanquianga NNP and the Malpelo FFS.
The protected area of Sanquianga NNP, located in the coastal zone, had the highest levels of abundance, Cetengraulis mysticetus being the most abundant species, and one of the zone’s most important from an ecological and economic point of view, as it is at the bottom of the food web and, in the past, was also the main raw material for the fish meal and fish oil industries (Beltrán-León, 1992; Beltrán-León et al., 1994; Zapata et al., 2013). Of the three areas, the Gorgona NNP displayed the greatest levels of diversity and richness, a finding that might be expected for an intermediate zone, as species from the coastal and the oceanic zones may be present there, though at lower levels of abundance. The predominant species was the ecologically important Bregmaceros bathymaster. The Malpelo FFS, located in the oceanic zone, was less abundant in larvae than Gorgona and Sanquianga, which is normal for tropical oceanic oligotrophic systems, that display lower levels of abundance. The dominant species was the ecologically important Vinciguerria lucetia.
It is probable that the drastic reduction in the abundance of eggs and fish larvae in Malpelo in November reflected the response of organisms to the high temperatures that characterized the zone in 2009 (> 28.5 °C), as recorded in the July 2009 Boletín de Alerta Climática (Climate Alert Bulletin) (BAC, 2009), which reported on an El Niño event that lasted into the first quarter of 2010. In the bulletin, the Centro de Control de Contaminación del Pacífico (Pacific Pollution Control Center, CCCP) reported a positive anomaly of 0.72 °C compared to the historical average for the monitoring it carried out in July 2009.
The impoverishment of pelagic habitats during the warm phase of the El Nino Southern Oscillation (ENSO) is one of the effects that has been most frequently described in the literature (Martínez-Aguilar et al., 2010). In 2009, eggs of the Engraulidae family were found in great abundance in Gorgona but were decidedly scarce around Malpelo (to the west at night and to the south during the day). It is worth stressing that this was the first time that individuals of this family were collected in Malpelo, which - because of its oceanic location - is not the typical place to find a family that is considered coastal pelagic typically found between 8 and 12 km offshore, at depths not exceeding 25 m (Chirichigno, 1998). This is likely explained by possible changes in the system of currents in the Colombian Pacific, which enabled members of this family to disperse to zones beyond their habitual distribution areas. This is consistent with the suggestion by López-Peralta (1984), who argued that, as fish eggs float passively, they should be expected to occur in numbers in a part of the ocean influenced by the pattern of surface circulation, itself affected by the Colombian current, which is strongest in November (trade winds blowing from the south to the northwest), and by the weakening of the Panama Jet during the rainy season (May-November), while the Colombian Pacific circulation is dominated by the formation of an anticyclonic rotation (Rodríguez-Rubio et al., 2003; Devis-Morales et al., 2008). In general terms, seasonal oscillations in the trade winds have been found to constitute the principal atmospheric driver modulating the pattern of mesoscale circulation and regional productivity (Rodríguez-Rubio and Giraldo, 2011). This necessarily implies the transport of eggs away from the coast, increasing their density in the oceanic zone. Similarly, it is possible that the Engraulidae family was responding to the anomalous environmental changes (high temperatures) it faced (Beltrán-León, 2002).
The NMS analysis of the community was very consistent, in that it made it possible to visualize the grouping of the stations and species by area, justifying the choice of locations, as follows: the coastal zone of the Sanquianga NNP where Cetengraulis mysticetus dominated, the Gorgona NNP represented by Bregmaceros bathymaster and the oceanic zone of the Malpelo FFS where Vinciguerrria lucetia was the most abundant. These findings illustrate that differences in the environmental parameters influence the distribution of the larvae as well as the families that were exclusive to each zone.
CONCLUSIONS
For many fish species, November 2009 was not a significant spawning month and yolk-sac larvae were not abundant in the protected areas. The greatest abundance of larvae in the Sanquianga NNP, in the coastal zone, came from Engraulidae, while the Gorgona NNP presented the greatest abundance of fish larvae and the Malpelo FFS the lowest abundance of eggs and fish larvae, probably the result of the response of organisms to the high temperatures experienced during November 2009.
The principal families captured were, by area, Engraulidae with Cetengraulis mysticetus (Sanquianga NNP), Bregmacerotidae represented by Bregmaceros bathymaster (Gorgona NNP) and Phosichthyidae in the form of Vinciguerria lucetia (Malpelo FFS). The NMS analysis of the community was very consistent, confirming the separation of the areas into coastal, intermediate and oceanic zones (respectively, the Sanquianga and Gorgona NNPs and the Malpelo FFS), demonstrating in this way the differences in the environmental parameters that influenced the distribution and exclusivity of larvae across the different families. The average temperatures found in the Malpelo FFS suggest the presence of a positive thermal anomaly, showing that this exerted influence on the distribution pattern and reduced abundance of ichthyoplankton. In this zone, eggs of pelagic coastal Engraulidae were also found, probably as a result of changes to the system of currents in the Colombian Pacific, which allowed members of the family to disperse to zones in which they are not usually found.











 text in
text in