The deterioration of water quality in coastal waters is a growing problem. Uncontrolled urban and industrial sewage discharges plus agricultural runoff worsen the quality of coastal waters (Aguilera et al., 2019; Devlin et al., 2020; Zhou et al., 2021). Land and ocean-based anthropogenic activities impact not only the quality of coastal and ocean waters (Azrina et al., 2006; Puccinelli et al., 2016; Häder et al., 2020;), but most marine ecosystems. The protected landscape of Galeta Island is located on the Caribbean coast of Colon province, Panama (Wang et al., 2008). Punta Galeta is approximately 8 km northeast of the city of Colón, near one of the entrances of the Panama Canal (González et al., 2019; Broce et al., 2022). The protected landscape and its management do not address all existing and emerging threats to marine systems (Halpern et al., 2010; Broce et al., 2022). Pollutants from human activities, drained from land and ocean spills of chemical or oil affect the marine ecosystems (Puccinelli et al., 2016; Aguilera et al., 2019), including the quality of water, sediments, and marine organisms (Angelidis and Aloupi, 2000; Zhang et al., 2017; Cebe and Balas, 2018). On the other hand, the exploitation of marine resources represents a significant threat for the marine environment and causes a continuous degradation of the ecosystem (Tonacci et al., 2018). From various points of view, it is uttermost to measure and monitor the water quality to assure minimal negative impacts on marine ecosystems.
To try to mitigate man-made effects, several initiatives such as national and international research projects, and regulations have been promoted in the last decades. All these initiatives highlight the need for preventive actions that include continuous monitoring programs in marine ecosystems (Azzellino et al., 2012; Tonacci et al., 2018). Among these initiatives, was the Red Mesoamericana de Calidad de Aguas (Remeca) who was driven by Mexico and involved the countries of: Guatemala, Belize, Honduras, El Salvador, Nicaragua, Costa Rica, Panama, Colombia, and Dominican Republic. The main goal of Remeca was to unify techniques of water sampling and analysis within the region and to establish water quality variables as possible indicators of climate change. In Panama, within the Remeca initiative, additionally to measuring water quality parameters, biological monitoring was included to assess the water quality of the marine ecosystem of Punta Galeta. Sampling area was limited to 9.40° N, -79.86° W (Figure 1). The sampling area included three subsampling stations located in a seagrass system nearby a mangrove forest in shallow waters with a water depth below 2 m. Sampling campaign took place in October 2014 and was carried out weekly in a 30-day period. Physicochemical parameters (i.e., pH, water temperature, salinity, dissolved oxygen) were measured using a multiparameter HACH equipment, model HQ40d. Four replicates of surface water samples from each site were collected for analysis of biological oxygen demand (BOD), fecal coliforms, nitrate, and phosphate. Samples for the analysis of chlorophyll-a were collected and determined in accordance with method EPA-446.0. This method determines chlorophylls a (chl a), b (chl b), c 1 + c 2 (chl c1 + c2) and pheopigments of chlorophyll a (pheo a) in marine and freshwater phytoplankton. Chlorophyllide a is determined as chl a. Visible wavelength spectrophotometry is used to measure the pigments in sub-parts per million (ppm) concentrations. The trichromatic equations of Jeffrey and Humphrey (1975) are used to calculate the concentrations of chl a, chl b, and chl c 1 +c 2 .
Modified monochromatic equations of Lorenzen (1967) are used to calculate pheopigment-corrected chl a and pheo a (Lorenzen, 1967). The concentration of nitrate was determined by the method of cadmium reduction (i.e., HACH 8039) for fresh, wastewater and seawater. Cadmium metal reduces nitrate in the sample to nitrite. The nitrite ion reacts in an acidic medium with sulfanilic acid to form an intermediate diazonium salt. The salt couples with gentisic acid to form an amber colored solution. The measurement wavelength is 500 nm for spectrophotometers or 520 nm for colorimeters. For quality control, the instrument was calibrated with nitrate standards solutions. Standard addition methods and standard solution methods were used to validate the test procedure, regent, and instrument. Concentrations of phosphate were determined by the method of ascorbic acid (i.e., HACH 8048) for fresh, wastewater and seawater. Orthophosphate reacts with molybdate in an acid medium to produce a mixed phosphate/molybdate complex. Ascorbic acid then reduces the complex, which gives an intense molybdenum blue color. The measurement wavelength is 880 nm for spectrophotometers. For quality control, the instrument was calibrated with standards solutions. Standard addition methods and standard solution methods were used to validate the test procedure, regent and instrument. BOD and fecal coliforms were determined in accordance with the standard methods procedures (Garay Tinoco et al., 2003). Descriptive statistical analysis and a one-way variance analysis (ANOVA) were done to assess environmental variability.
Water quality index (WQI) was calculated with values of temperature, pH, dissolved oxygen, turbidity, phosphate, nitrate, biological oxygen demand (BOD5), and fecal coliform. The index is calculated from Q value and weight factor W, where Q indicates the level of water quality relative to any single parameter and the weight factor represents the relative importance of the single parameter to the overall water quality (Jahan and Strezov, 2017). Biological monitoring included the sampling of benthic macroinvertebrates along the seagrass field and sheltered from the action of waves, between the mangrove forest and coral reef and a water depth < 2 m (Figure 1). The sampling area was subdivided into three sub-samples. Each sub-sample had a catching area of 1 m2 over the seagrass habitat. Three replicates were done at each sub-sample. Bottom-sediment was extracted with a Hope type corer sampler of 5 cm diameter and 15 cm depth. Organisms were carefully separated from the sediment with hands to avoid any loss of material and preserved in a solution of 94 % ethanol, after the extraction (Azrina et al., 2006).
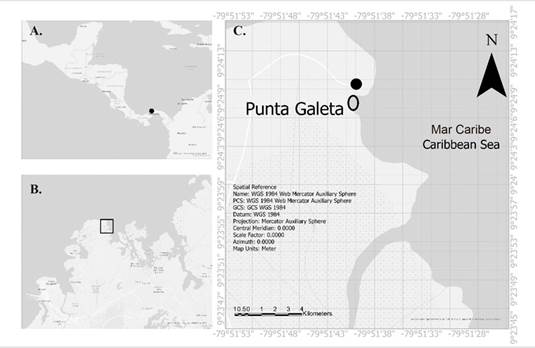
Figure 1 Study Area. A. Location of Panama in Central America, B. Map of Punta Galeta, Province of Colon, Panama. C. The STRI Marine Laboratory (black dot), and the ellipse denotes the sampling area.
Throughout the results section, reported variables will be compared to international regulations and/or previous research studies because Panama has no regulation for quality of marine waters. Reported values of surface temperature coincide with regional temperature values, which vary between 25 and 29 °C (Beier et al., 2017). Warmer waters are observed south of 12 ° N in the Panama-Colombia regions of the Caribbean Sea. Minimum values of salinity indicate low salinity waters (25), due to rainfall and the influence of river discharge (Table 1), and maximum values (35) indicate saltier water, within normal ranges (Chollett et al., 2012; Beier et al., 2017).
Recorded values ranged of 1 to 7 NTU of turbidity at Punta Galeta do not interfere with marine organisms and their habitats. Turbidity values are considered low compared to 30 NTU which is a level sufficient to alter the visual acuity of marine organisms (Lunt and Smee, 2020). Values of dissolved oxygen were between 7.9 to 13.1 mg L-1 and values of pH were between 7.8 to 8.4 (Table 1). Both parameters are suitable for preserving aquatic life (Van Woesik et al., 2012; Jordán-Garza et al., 2017). BOD5 values are within 0.1 to 0.2, that are included in the permissible values for the conservation of aquatic life (Alfayate Blanco et al., 2004; Garrison et al., 2021; Oyeniran et al., 2021). Values of nitrate recorded were in the range of 0.2-0.4 mg L-1.
Table 1 Physicochemical and biological parameters (mean values and standard deviation) and Water Quality Index for sea water in Punta Galeta. Water quality index legend: 91-100 (very good), 71-90 (good), 51-70 (moderate), 26-50 (bad), 0-25 (very bad). (Gupta et al., 2003; Nikoo et al., 2011).
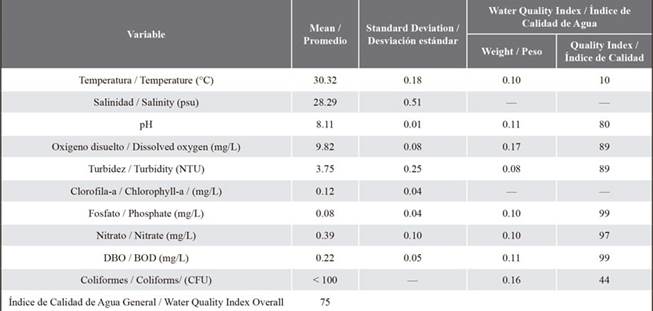
According to the criteria for water quality, nitrate values exceeded the values for the conservation of life which is 0.04 mg L-1. Concentrations of phosphates were within the range of 0.04-0.12 mg L-1, exceedingly the Mexican ecological criteria for water quality. Values for Chlorophyll-a were within the range 0.08-0.163 mg L-1, there is no standard for the quality of marine waters; however, this parameter is a good indicator of primary production. An enhanced chlorophyll-a (Chl-a) concentration is associated with the tropical instability wave (TIWs) in the Equatorial Pacific Ocean (Shi and Wang, 2021).
Chl-a concentration is also critical when conducting large-scale modelling studies in the tropical Pacific. The Chl-a variability driven by the TIW also influences both the intensity of TIW and the large-scale of the sea surface temperature (SST) in the tropical Pacific Ocean. In fact, positive feedback on the ENSO is observed due to the TIW-induced Chl-a effect (Shi and Wang, 2021). Fecal coliforms measurements were low during the sampling period, showing no contamination due to these bacteria (<100 CFU 100 ml-1). According to the calculated overall water quality index (WQI) for Punta Galeta, which included eight of the parameters mentioned above, water quality is good (75).
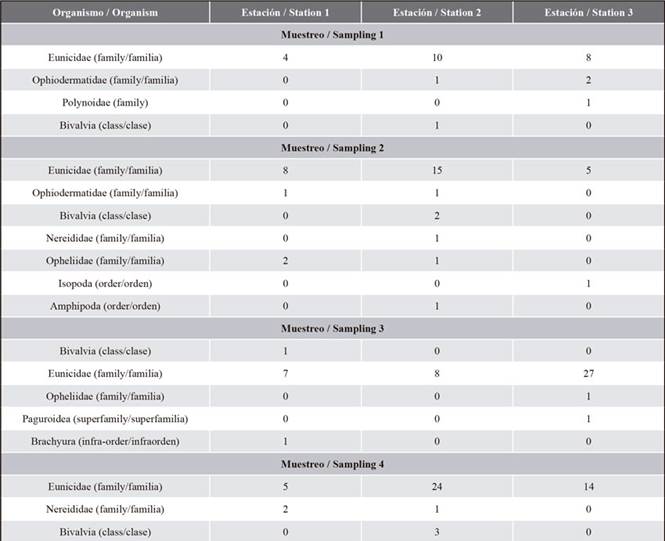
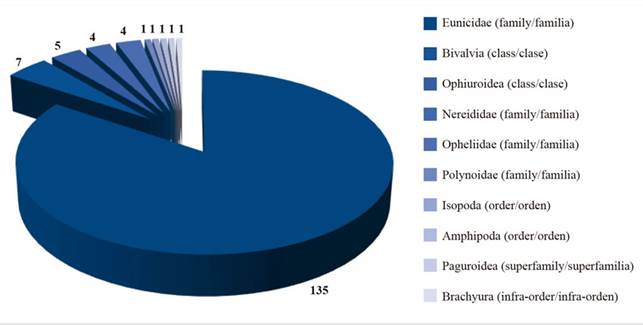
A total of 160 benthic organisms were captured, 27 organisms in the first sampling, 38 in the second sampling, 46 in the third sampling and 49 in the fourth sampling. The organisms were identified among the taxonomic classifications of class, order and family. The taxonomic classification included class (Bivalvia), order (Isopoda, Amphipoda, Brachyura) and family (Paguroidea, Eunicidae, Ophiodermatidae, Polynoidae, Nereididae, Opheliidae). Ten taxa were identified from the 160 collected benthic organisms (Table 2). Previous studies reported similar taxa in the seagrasses of the Caribbean of Panama (Marshall, 1991). The most abundant taxon was polychaetes with 144 organisms (90 % of the total), mollusks with 7 organisms (4 % of the total), crustaceans and echinoderms with 4 and 5 organisms, representing 3 % of the total collected organisms (Figure 2).
Seawater quality in Punta Galeta was based on the water quality index and results from a short-term biological study. Presence of identified benthic macroinvertebrates agree the richness of coastal tropical sites, especially due to the adaptation of species of these communities to the environmental conditions of this zone (Medeiros et al., 2016). The number and type of macroinvertebrates were partly determined by the wave action, the range of salinity and temperature (Horrigan et al., 2005; Wolf et al., 2008; Medeiros et al., 2016; De Marchi et al., 2018), granulometry and availability of organic matter (Rodrigues et al., 2006; Beghelli et al., 2012), and by the good quality of seawater (Lock et al., 2011; Fierro et al., 2019). Even though concentrations of nitrate and phosphate were above the recommended value (Ma et al., 2020), the reported values do not restrict the abundance of benthic macroinvertebrates as reported by Peng et al. (2020) in rivers of China with increased levels of nutrients.
Long-term studies with in-situ continuous measurements of physicochemical variables are required to understand the seasonality changes in water quality and its subsequent link to the abundance and richness of the benthic community. These biological assemblages have been used as an effective tool to evaluate sewage contamination (Moreno and Callisto, 2006) and quality of freshwater streams. This tool could be also an effective bio-indicator for marine environments, due to their sedentary lifestyle, large size, relatively long-life span and variable tolerance to human induced pressures (Jordan and Smith, 2004). Obtained results from this study are relevant in Panama to define the threshold of physical parameters where polychaetas do not survive. Monitoring of physicochemical parameters and biological studies reflect better the water quality of the ecosystem. Results from a more comprehensive study can work as a baseline for marine environment legislation in Panama.
Methodologies that include biological monitoring techniques along with analytical physicochemical procedures can better describe water quality and knowledge of the ecological status of the site, uttermost facts for scientific based legislations for the protection of marine environments.











 text in
text in