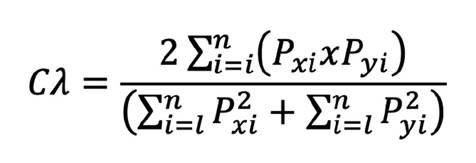INTRODUCTION
Recreational fishing is an activity that involves attracting, capturing, and releasing fish. In Colombia, it is practiced in 80 % of the national territory, both in freshwater and marine environments (Lasso et al., 2019). In the Colombian Caribbean, artisanal recreational fishing is a dual activity: it is practiced for livelihood and tourism purposes. Artisanal fishermen use traditional methods, such as live bait, to catch fish such as snooks, snappers, and Spanish mackerels (Lasso et al., 2019). These fish are sold in local markets or consumed at home. Sport fishing is also a significant touristic activity in the Colombian Caribbean; both national and foreign tourists visit the region to engage in recreational fishing (Lasso et al., 2019). This activity generates economic income for local communities and contributes to the development of sustainable tourism. Among the species most caught by recreational fishing in the Colombian Caribbean are little tunny, albacore, dorado, and other small pelagic fish (Paramo et al., 2019).
The little tunny, Euthynnus alletteratus (Rafinesque), and the albacore, Thunnus alalunga (Bonnaterre), are epipelagic, neritic tuna. They are highly migratory species, distributed across the Atlantic ocean (E. alletteratus) or circumtropical areas (T. alalunga) (Vieira et al., 2021), inhabiting temperate and subtropical waters in their juvenile stages. Once they reach the adult stages, they are found in subsurface waters and at depths of around 200 m (Bertrand et al., 2002; Vieira et al., 2021). These species are opportunistic predators and eat a great diversity of prey. The diet of the little tunny and the albacora has been described in different parts of the world (Consoli et al., 2008; Young et al., 2010; Varghese et al., 2014; Varela et al., 2017), but there is still a limited number of studies on their feeding habits, and few works have focused on the changes in their diet with respect to climatic seasons (García and Posada, 2013). In general, these fish feed mainly on crustaceans, fish, and cephalopods. In some cases, the main prey found in their stomachs are bony fish remains, fish such as Engraulis japonicus, and cephalopods such as Gonatopsis borealis (Ruiz-Pérez et al., 2016). All this, according to these species’ relative importance index (RII) (Pinkas, 1971; Martínez-Juárez et al., 2023).
Some species of tuna, such as the small tuna, are abundant in the Mediterranean Sea, with great commercial and ecological importance, as they are predators of the upper portion of the trophic web (Báez et al., 2021). These tunas are coastal species, so they are caught by several types of fishing gear. In the case of Colombia, they are caught by small artisanal boats using nets and rods. This fishery product is used mainly for own consumption. For the Colombian Caribbean, studies on the feeding habits of these two species are scarce (García and Posada, 2013). The diet of E. alletteratus, based on stomach content analyses, is mainly made up of prey items from the families Clupeidae (Harengula sp., Sardinella aurita, Opisthonema oglinum) and Carangidae (Decapterus sp.) (Moreno, 1986). Another study shows that the diet of E. alletteratus in the Colombian Caribbean mainly comprises species from the families Clupeidae (Jenkinsia lamprotaenia and S. aurita), Carangidae (Decapterus sp.), and Mugilidae (Mugil curema and Mugil sp.) (García and Posada, 2013; Posada, 2017). In the case of T. alalunga, no studies have been conducted with regard to feeding habits in this region. The objective of this research was to evaluate the feeding habits of E. alletteratus and T. alalunga through a quantitative analysis (numerical, gravimetric, and relative frequency) of their diets and diversity, amplitude, and trophic similarity for the region of Santa Marta in the Colombian Caribbean.
STUDY AREA
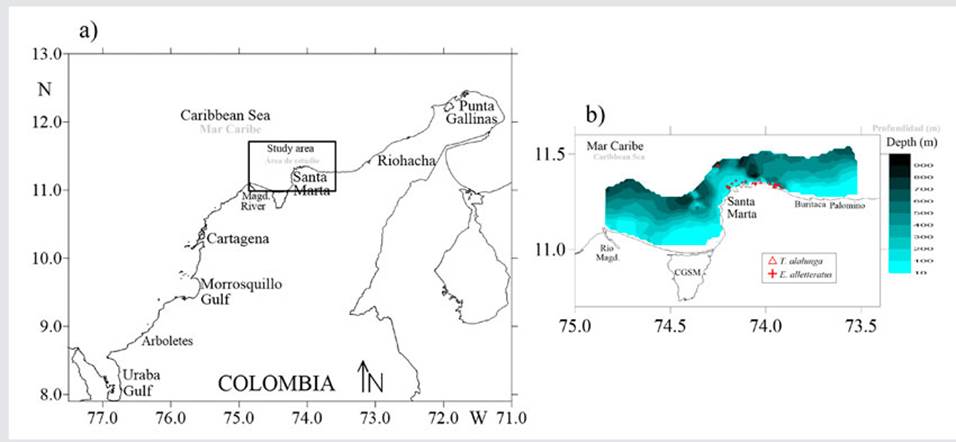
Santa Marta is located on the Caribbean coast of Colombia, in the department of Magdalena (Figure 1). This area is governed by two climatic periods: a dry season and a rainy season, influenced by the north - south displacement of the intertropical convergence zone (ITCZ) (Franco-Herrera, 2005; Paramo et al., 2009). When the ITCZ moves to the south, the northeast trade winds influence the region (dry season), and, when the ITCZ moves to the north, the winds are lower, promoting precipitation (rainy season) (Franco-Herrera, 2005).
MATERIALS AND METHODS
Stomachs of E. alletteratus and T. alalunga were collected between March 2018 and April 2019 off the coast of Santa Marta. The specimens were captured during daytime using fishing rods with live bait and artificial lures, a common technique in recreational fishing (Klett-Traulsen et al., 1996; Lloret et al., 2008). With the purpose of identifying seasonal variations in the feeding habits of the aforementioned species, temperature and salinity data were obtained in situ using a CTD (Castaway). In addition, monthly records of sea surface temperature (SST), sea surface salinity (SSS), and winds were obtained from satellite data. The SST information was captured on a daily basis via the MODIS-Aqua sensor from the Ocean Color website (http://oceandata.sci.gsfc.nasa.gov), with a spatial resolution of ~ 4 km. The surface salinity was obtained from the Marine Environmental Monitoring Service (Copernicus) of the European Commission (http://marine.copernicus.eu), with a spatial resolution of ~ 9 km. Finally, the wind information (m/s) came from the North American Regional Reanalysis database (NARR, North American Regional Reanalysis; ftp://ftp.cdc.noaa.gov/Datasets/NARR), with a spatial resolution of 0.3° (~32 km).
Once captured, identified, and stored in ice for conservation and transport to the laboratory, each individual was sexed, measured (total length, TL, to the nearest mm) with a measuring tape, and weighed (g) using a digital weight with an accuracy of 0.1 g. Subsequently, the stomach was extracted, preserved, and stored in 10 % formaldehyde (Hyslop, 1980). Preys were extracted from the stomachs, counted, weighed, and identified, depending on the state of digestion (Clothier, 1950), to the lowest taxonomic category using the specialized literature (Manning, 1969; Méndez, 1981; Cervigón, 1992; Fischer et al.,1995; Chirichigno and Vélez, 1998). The minimum sample size was determined by means of prey accumulation curves, which were elaborated based on the number of stomachs analyzed via the EstimateS software, version 9.1.0 (Villareal et al., 2004; Colwell et al., 2012), with 999 permutations. To determine the representativeness of the sample size, the coefficient of variation was employed. The stomachs were valid when at least five sample units were found below 5 % (Ferry et al., 1997). Additionally, linear regression was performed to confirm that the slope was not significantly different from zero (Bizarro et al., 1997). When these two premises were fulfilled, the sample was considered to be representative. Three relative measures of prey quantity were estimated: numerical (% N), gravimetric (% G), and frequency of occurrence (% OF) (Hyslop, 1980). The importance of each item prey was calculated using the relative importance index (RII), which uses the three measures mentioned above (Pinkas et al., 1971). The standardized equation of the Levin index was implemented to determine the niche breadth, and diet diversity was assessed by calculating the Shannon-Wiener index (H’) (Krebs, 1999).
The differences in the diets of E. alletteratus and T. alalunga (based on prey abundance) were calculated for each season and size class via the chi-squared test and cluster analysis. The Bray-Curtis index was applied to assess the degree of similarity in prey abundance. The data were log-transformed (log × + 1) to reduce the influence of zeros in the more abundant species (Paramo et al., 2012).
The Morisita-Horn index was used to determine the dietary overlap (Cλ) (Krebs, 1999).
This overlap is determined based on the index values: when the values are between 0 and 0.29, they indicate no overlap in the trophic component, and values of 0.6 to 1 show a biologically significant overlap (Krebs, 1999).
RESULTS
Four climatic seasons related to the intensity of the winds, temperature, and salinity were found. The period of greatest intensity was between December and March, with values between 7.2 and 11.1 m/s. This corresponds to the major dry season (MaD), which is associated with lower sea surface temperature (24.5-26.8 °C) and higher salinity values (35.8-36.8). The minor dry season (MinD), between July and August, exhibits medium wind intensity values ranging from 6.8 to 7.4 m/s, with sea surface temperature values between 26.7 and 27.8 °C and salinities of 35.5 - 36.1. The season with low-intensity winds (April to June) is associated with minor rainfall (MinR), with values between 4.0 and 7.8 m/s, sea surface temperatures between 26.5 and 27.7 °C, and salinities between 35.8 and 36.7. The season with the lowest wind intensity occurred between September and November (1.3 and 6.4 m/s), which corresponds to major rainfall (MaR), with the highest temperatures (27.9 and 29.0 °C) and the lowest salinity values (35.7 and 35.9) in the entire area (Figure 2).
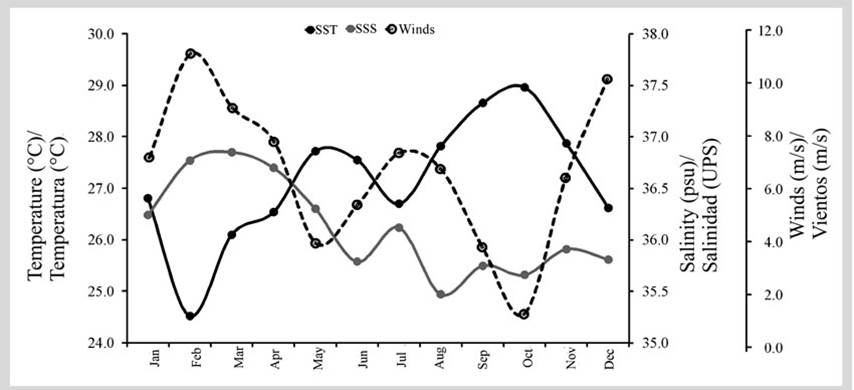
Figure 2 Monthly variation in SST, SSS, and wind intensity (m/s) in Santa Marta’s marine region, Colombian Caribbean. Major dry season (December - February), minor dry season (July - August), minor rainy season (April - June), and major rainy season (September - November).
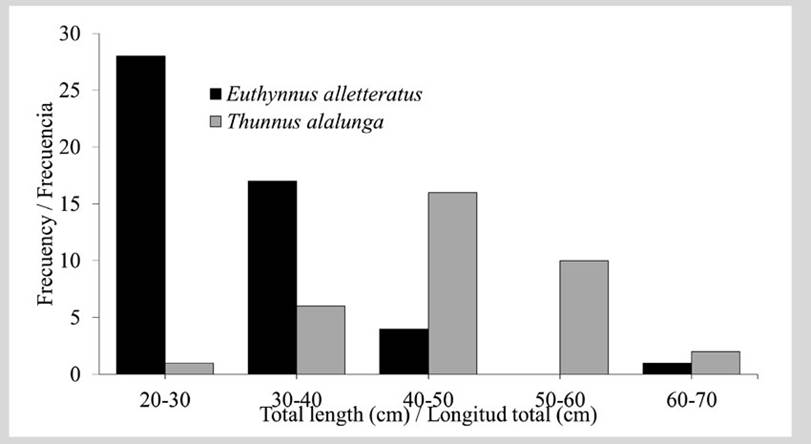
85 tuna stomachs were collected, 50 of E. alletteratus and 35 of T. alalunga. The size of E. alletteratus varied between 24.5 and 63.0 cm (mean: 31.96 ± 6.66 cm), while the sizes of T. alalunga were greater, ranging between 42.0 and 63.0 cm (mean 50.39 ± 6.43 cm). The two tuna species were grouped into five classes (20 - 30, 30 - 40, 40 - 50, 50 - 60, 60 - 70 cm) (Figure 3). Euthynnus alletteratus exhibited an OF of 58 % in the 20 - 30 cm class, 32 % in the 30 - 40 cm class, and only 8 and 2 % in the 40 - 50 and 60 - 70 cm classes. No individuals in the 50 - 60 cm class were captured (Figure 3). Thunnus alalunga showed a higher frequency in size classes 20 - 30 and 40 - 50 cm (29 % each), followed by class 30 - 40 cm (26 %), and the 50 - 60 (14 %) and 60 - 70 cm (3 %) classes.
85 stomachs were analyzed, and 91 % (77 stomachs) contained at least one prey. According to the accumulation curve of prey item species, E. alletteratus (Figure 4a) reached an asymptote with 40 stomachs, while T. alalunga reached it with 30 stomachs (Figure 4b).
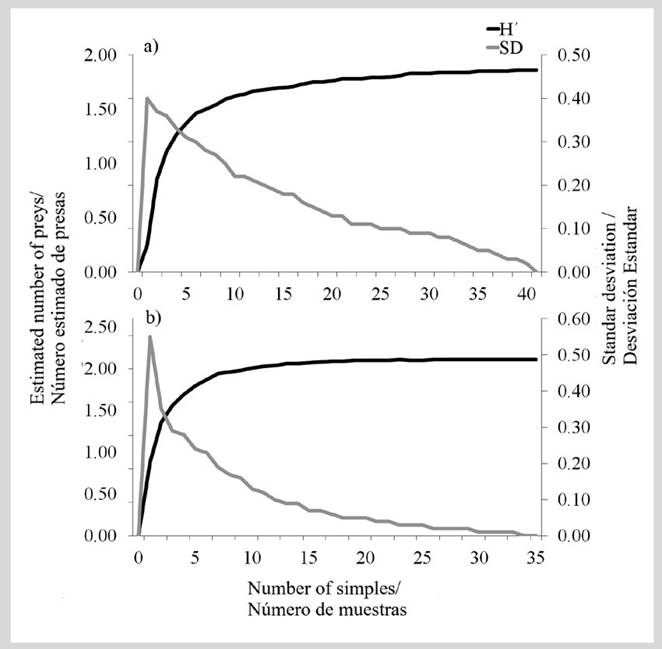
Figure 4 Prey accumulation curve and standard deviation of a) bonito (E. alletteratus) and b) albacore (T. alalunga) in Santa Marta
In the stomachs analyzed, 494 preys were found, which allowed identifying 14 food items, out of which five belonged to the class Malacostraca, five to the class Actinopterygii, three to the class Cephalopoda, one to Gastropoda, and one to Algae. Megalopa larvae were the dominant prey item of the two species. Sardinella aurita (43 %) and Teleostei remains (28 %) were the dominant prey items in the stomach content of E. alletteratus. Megalopa larvae (57 %) and Teleostei remains (10 %) were the dominant prey items in the stomach content of T. alalunga. Plastic residues (0.0027 g) (Figure 5) were observed in the stomach of one E. alletteratus individual.
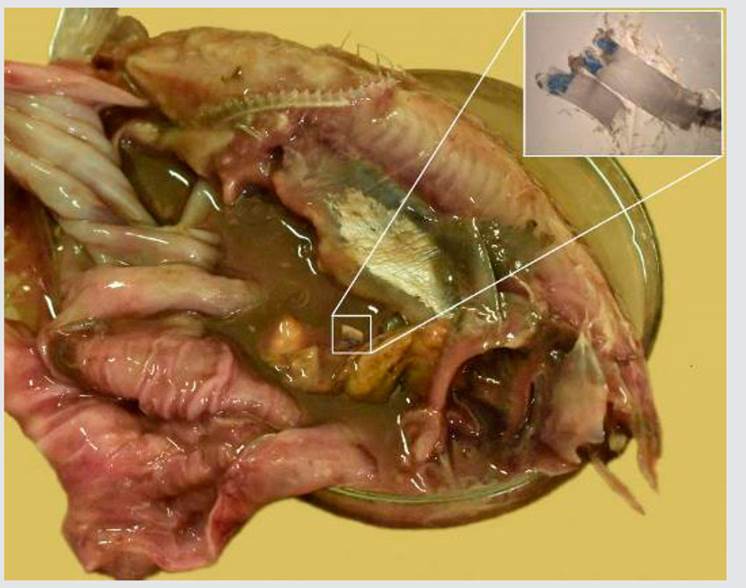
Figure 5.Plastic residues found in the stomach contents of bonito E. alletteratus from Santa Marta. Collection code PESD - 0021, location: 74°1.6’36” W and 11°20.8’48” N.
At least one prey was found in 91 % of the stomachs of male and female E. alletteratus individuals, with only 9 % of the stomachs empty. Out of the undetermined individuals’ stomachs 40 % was full and 60 % empty. The diet of male specimens consisted of eight food items, with S. aurita being the most representative. In the case of the females, the diet consisted of six food items, also with S. aurita as the most representative. The diet of the undetermined individuals was composed of five food items, with the prey Teleostei remains being the most representative. At least one prey was found in 95 % of the male stomachs of T. alalunga, and 5 % of the stomachs were empty. However, in the females and undetermined individuals, all stomachs were full. The male diet consisted of eight food items, with megalopa larvae being the most abundant item. In the case of females, the diet was composed of 11 food items, with larvae as the most abundant prey. The diet of the undetermined individuals was composed of seven food items, with Megalopa and Stomatopoda larvae as the most abundant prey.
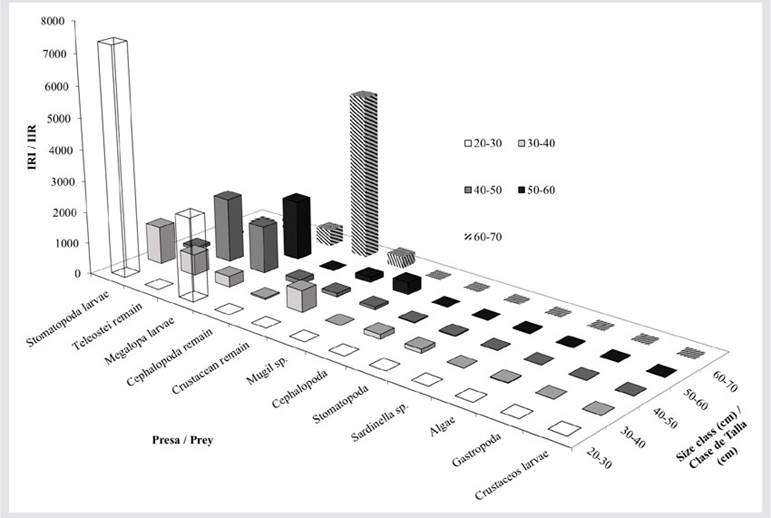
The RII of the tunas allowed identifying Teleostei remains (994.01), megalopa larvae (865.29), and S. aurita (247.11) as the main prey. Four items were determined as secondary prey: Stomatopoda larvae (102.64), crustacean remains (90.30), Mugil sp. (71.92), and Diapterus sp. (49.40). For E. alletteratus, Teleostei remains (1460.65) and S. aurita (1179.70) were the most important prey (Figure 6). Six secondary prey items were found: megalopa larvae (190.86), Diapterus sp. (152.56), Stomatopoda larvae (68.20), Clupeidae (29.24), and Mugil sp. (07/28). For T. alalunga the main prey were megalopa larvae (1367.19) and Teleostei remains (840.94), and the secondary prey were Mugil sp. (199.02), crustacean remains (155.85), Stomatopoda larvae (152.63), and Cephalopoda remains (23.48).
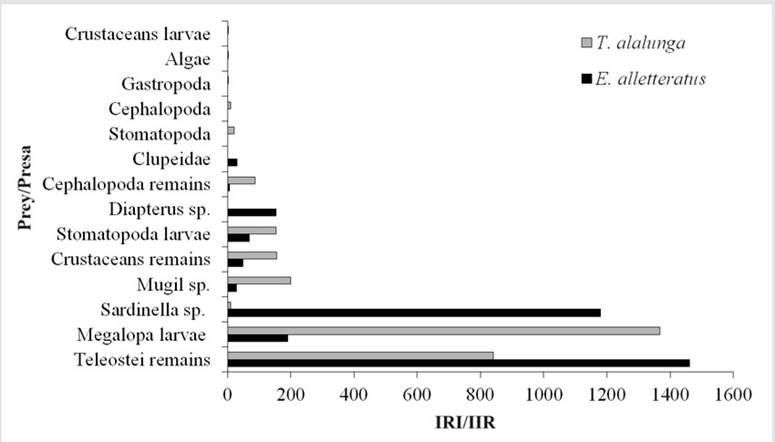
Figure 6 RII of the prey categories found in the stomach contents of E. allatteratus and T. alalunga. RII > 200, main prey; RII 21 - 200, secondary prey; RII 0 - 20, occasional prey.
Based on the RII, the most important prey for E. alletteratus were fish and Teleostei remains (Figure 7). The diet of bonitos in class 20 - 30 cm was dominated by S. aurita and Teleostei remains, with lower RII values for megalopa larvae, Clupeidae, crustacean remains, and Stomatopoda larvae. In size class 30 - 40 cm, the diet was composed of seven food items, with Megalopa, Teleostei remains, and Mugil sp. being the most abundant, along with Stomatopoda larvae, crustacean remains, Clupeidae, and Cephalopoda remains. In size class 40 - 50 cm, the diet consisted of three food items: Stomatopoda larvae, Teleostei remains, and megalopa larvae. Finally, in size class 60 - 70 cm, the diet was composed exclusively of Diapterus sp. and Teleostei remains (Figure 7).
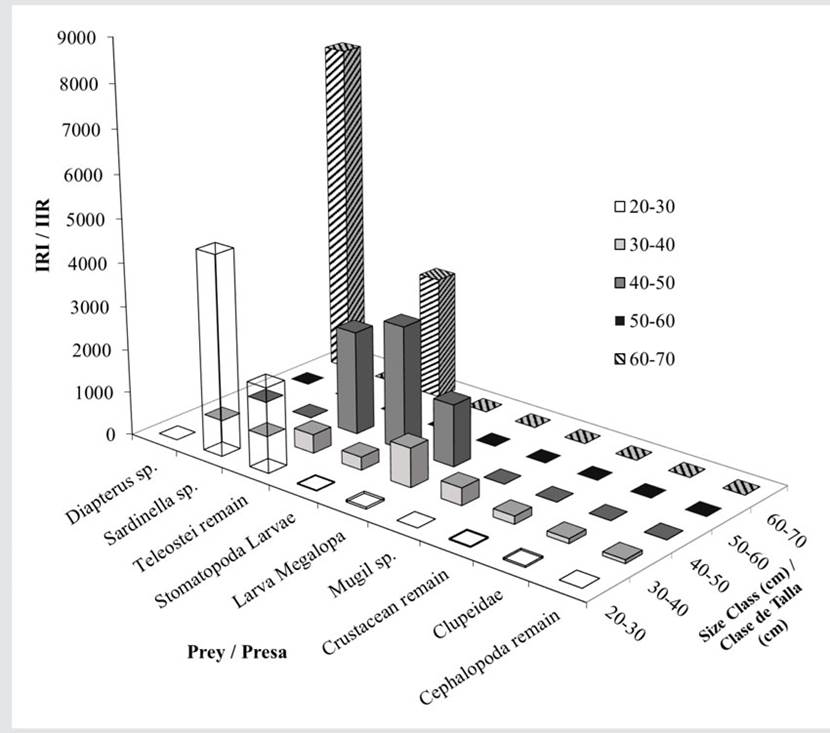
Figure 7 RII of the prey categories in the different size classes of E. alletteratus and T. alalunga. RII > 200, main prey; RII 21 - 200, secondary prey; RII 0 - 20, occasional preys.
The prey with the highest RII for T. alalunga were Stomatopoda and megalopa larvae and Teleostei, Cephalopoda, and crustacean remains (Figure 8). In size class 20 - 30 cm, the prey consisted only of Stomatopoda and megalopa larvae. The size class 30 - 40 cm was associated with Stomatopoda and crustacean and Teleostei remains, with little representation of megalopa larvae, Cephalopoda, and Stomatopoda and Cephalopoda remains. The prey in the 40 - 50 cm class was mainly composed of Teleostei remains and megalopa larvae, with very low values of crustacean remains, Stomatopoda larvae, Mugil sp., Cephalopoda remains, Stomatopoda, Cephalopoda, Sardinella sp., and crustacean larvae. In the 60 - 70 cm class, the greatest contribution of prey corresponded to Cephalopoda remains, followed by megalopa larvae and Teleostei and crustacean remains (Figure 8).
The dendrogram with the standardized prey abundance values (log x + 1) of E. alletteratus showed the presence of three main groups for the different size classes (55 % similarity), indicating maximum similarities between the diet of the 30 - 40 and 40 - 50 cm size classes (Figure 9a). The diet of size classes 20 - 30 and 60 - 70 cm was distributed in different groups (< 60 %). The prey abundance dendrogram for T. alalunga showed the presence of four main groups in the different size classes (55 % similarity), indicating maximum similarities between the diet of size classes 40 - 50 and 50 - 60 cm (Figure 9b). The diet of the 20 - 30, 30 - 40, and 60 - 70 cm sizes classes was distributed in different groups (< 60 %).
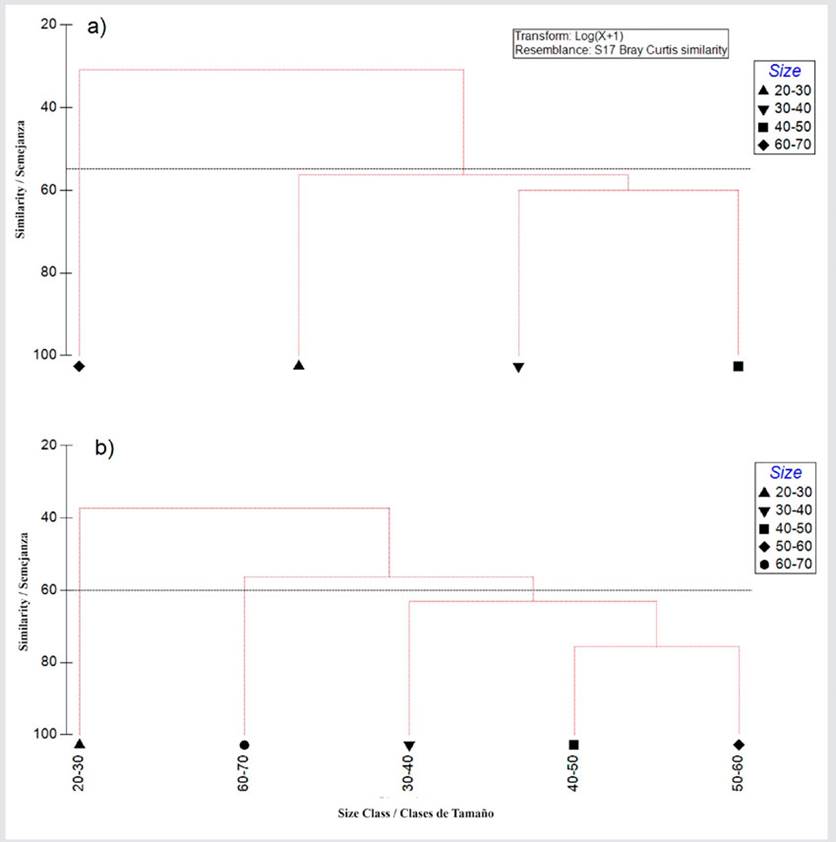
Figure 9 Cluster analysis dendrogram showing the Bray-Curtis similarity index for the size classes of a) E. alletteratus b) T. alalunga.
Differences in seasonal variation with regard to abundance were found in the stomach content of E. alletteratus (Table 1). Significant differences were found between the MaD and MinD seasons. The total number (% N) of prey such as S. aurita and Stomatopoda larvae shows a significant change over the seasons. Moreover, differences between MaD and MaR were found, as the % N of the prey in the phylum Arthropoda (megalopa and Stomatopoda larvae as well as crustacean remains) and Teleostei remains shows significant change (Table 1). Finally, significant differences were observed in MaD and MinR; the % N of prey such as S. aurita and megalopa larvae shows significant change. The diet of E. alletteratus was classified as generalist and not very diverse in all seasons (Table 2).
Table 1 Seasonal differences in the frequency of prey abundance for E. alletteratus and T. alalunga. Major dry (MaD), minor dry (MinD), major rainy (MaR), and minor rainy (MinR) seasons.
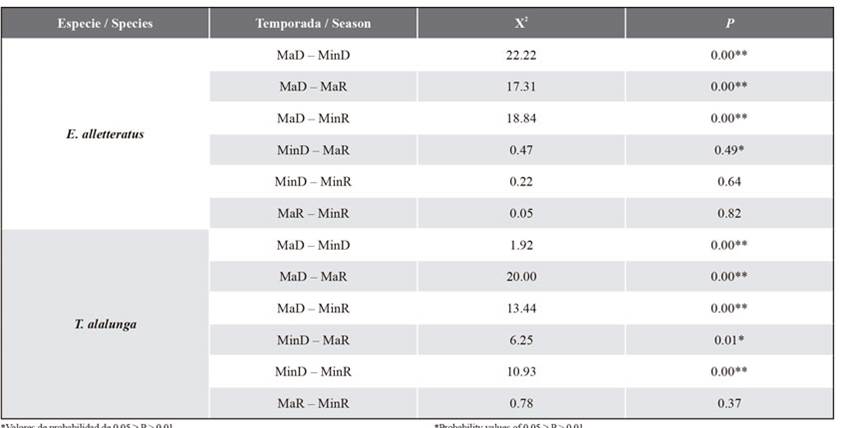
*Probability values of 0.05 ≥ P > 0.01.
**Probability values of 0.01 ≥ P.
Table 2 Dietary indices of prey groups found in the stomachs of Euthynnus alletteratus. % OF = frequency of occurrence, % N = numerical percentage, % P = gravimetric percentage, RII = relative importance index, B = Levin’s standardized indices, H’: Shannon diversity. Total values are indicated in bold.
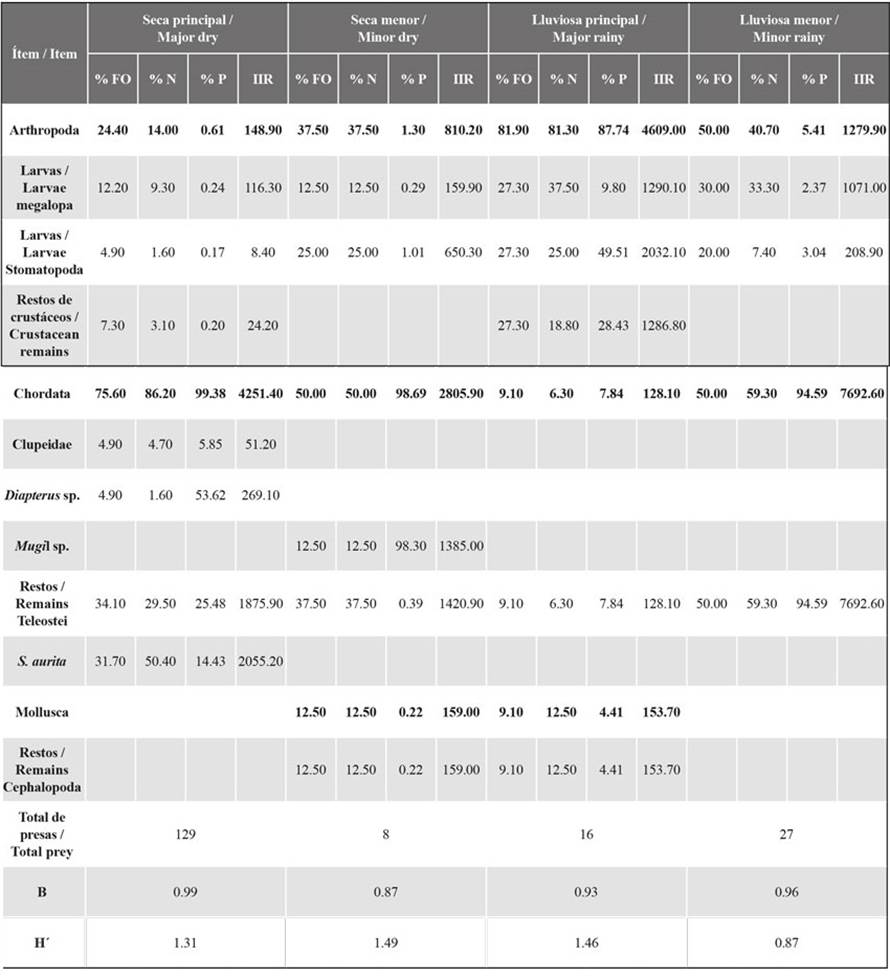
Seasonal variation differences regarding abundance were found in the stomach content of T. alalunga (Table 1). Significant differences between MaD and MinD were found, as the % N of prey such as megalopa larvae and Teleostei remains shows significant change over the seasons (Tablas 1 y 3). Significant differences were evidenced in MaD and MinR, with the % N of Teleostei remains showing significant change. Similarly, differences between MinD and MaR were found; the % N of crustacean remains, Stomatopoda larvae, and Teleostei remains also shows significant change. Finally, significant differences were evidenced in MinD and MinR, as the % N of megalopa larvae and crustacean and Teleostei remains shows significant change. The diet of T. alalunga was classified as generalist and not very diverse in all seasons (Table 3).
Table 3 Dietary indices of prey groups found in the stomachs of T. alalunga. % OF = frequency of occurrence, % N = numerical percentage, % P = gravimetric percentage, RII = relative importance index, B = Levin’s standardized indices, H’: Shannon diversity. Total values are indicated in bold.
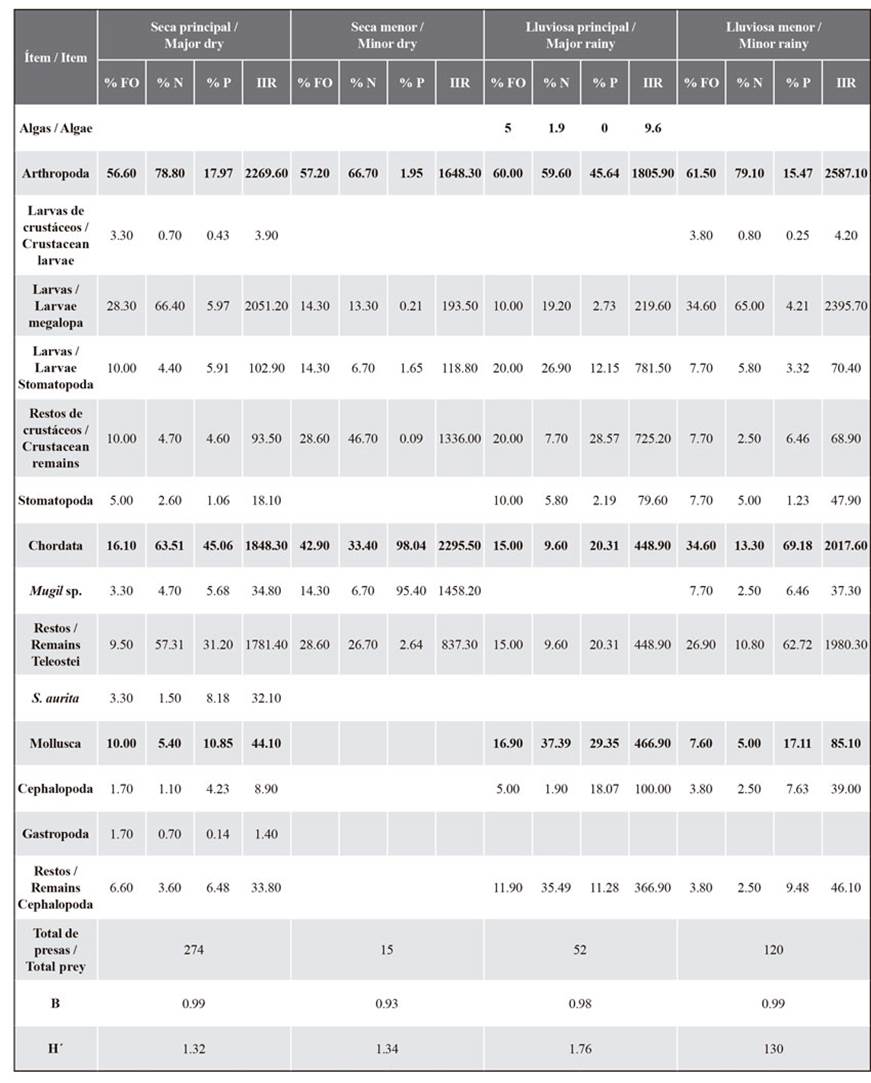
The dendrogram of the prey abundance values found in the stomach content of E. alletteratus shows the presence of six main groups for the different size classes (< 50 % similarity), indicating maximum similarities between MaR and MinD (Figure 10a). For February, March, August, and October, the diet of the bonito was distributed in different groups (< 50 %). The dendrogram of the prey abundance found in the stomach contents of T. alalunga shows the presence of seven main groups for the different climatic seasons (40 % similarity), indicating maximum similarities between MaD and MinR (Figure 10b). For January, April, August, September, and October, the diet of the albacore was distributed in different groups (40 %).
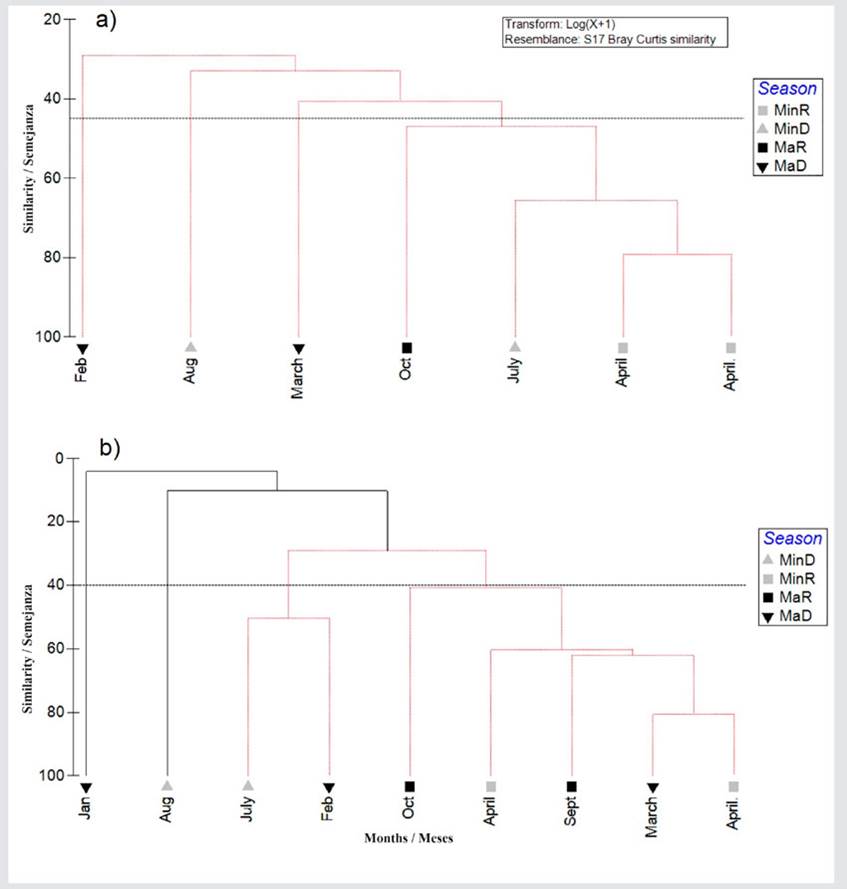
Figure 10 Cluster analysis dendrogram showing the Bray-Curtis similarity index for the prey found in the stomach contents of a) E. alletteratus and b) T. alalunga in different climatic seasons in Santa Marta.
The niche breadth showed that the tuna species have a generalist feeding behavior (Bi = 0.998). They feed proportionally from different types of prey. This behavior was found in both males (Bi = 0.996) and females (Bi = 0.995). The trophic niche breadth indices for the bonito (Bi = 0.993) and the albacore (Bi = 0.997) are high, which classifies them as generalist predators. In addition, the value of the Morisita-Horn index (Cλ= 0.5) does not show a high trophic overlap between the two species of tuna, which means that these predators are not feeding exactly on the same prey in the pelagic ecosystem.
DISCUSSION
There is limited research on the feeding habits of E. alletteratus and T. alalunga. Few works have focused on the changes in their diet in relation to climatic seasons. In general, tuna species such as bonito and albacore are opportunistic predators that consume a wide variety of prey (Varghese et al., 2014). This study is the first comparison of the diet composition between these two species in the Colombian Caribbean, which was based on characterizing the prey found in their stomach contents and on data regarding abundance, diet, feeding niche, and diet changes. A prey analysis revealed fish and crustaceans as the main diet components, suggesting some specialization. These findings are in line with the documented prey preferences of the genus Thunnus, including yellowfin tuna (T. albacares). These prey items rank high in frequency of occurrence (Baque-Monoscal et al., 2012; Battaglia et al., 2013; Logan et al., 2015; Zudaire et al., 2015; Navarro et al., 2017).
Most of the prey mainly stemmed from epipelagic habitats (S. aurita, Mugil sp., and crustacean larvae). The importance of small pelagic fish was evidenced, since Teleostei remains were found in most stomachs and contributed greatly to the diet of the studied organisms. In the two climatic seasons analyzed, no differences in the composition and diversity of the organisms consumed were evidenced, suggesting that it is necessary to continue conducting this type of research and increasing sampling efforts to be able to visualize dietary behavior for larger time scales and size structures. The diet composition can also be explained by different preferences due to ontogeny, i.e., it depends on the size structure, since juveniles and adults may have different energy requirements related to growth, nursery areas, and spawning migrations (Doncel and Páramo, 2010).
The diet of E. alletteratus was represented mainly by S. aurita (43 %) and Teleostei remains (27 %), as previously reported for the Colombian Caribbean, recording species such as Jenkinsia lamprotaenia and S. aurita, representatives of the family Clupeidae family, as the main prey (García and Posada, 2013). The feeding habits of E. alletteratus in the Santa Marta coastal area were low in terms of richness of food items. Similar results have been obtained for this species in the Colombian Caribbean, recording 9 - 15 food items, where fish has been the most important and representative element of stomach content in terms of weight (Moreno, 1986; García and Posada, 2013). Other studies have found a wider trophic spectrum (n = 59) in E. alletteratus in the central Mediterranean Sea, with fish being the most representative prey in terms of weight. Some feeding preferences were found which depend on the size classes of E. alletteratus, i.e., megalopa larvae and fish of the genus Sardinella in sizes of 20 - 40 cm.
Teleostei remains and fish of the genus Sardinella were the most important prey according to the RII of E. alletteratus. In the Colombian Caribbean, fish such as Decapterus sp., Harengula sp., J. lamprotaenia, and S. aurita have been recorded as the main prey in terms of gravimetric percentage (Moreno, 1986; García and Posada, 2013). On the other hand, in the Mexican Pacific, the diet of E. lineatus has been studied, with the most important prey being Opisthonema medirastre and Stomatopoda species according to the RII (Ruiz-Pérez et al., 2016). This result demonstrates the importance of epipelagic species in the diet of these organisms. The presence of Teleostei remains provides some insights on the studied species’ preferences. Teleostei likely contributes to the physiology of the fish in terms of energy (Varela et al., 2017; Romero et al., 2021).
57 % of the diet of T. alalunga was represented by Megalopa larvae, and 10 % by Teleostei remains, similarly to works reporting prey such as crustaceans. Nevertheless, the main food item was squid (Young et al., 2010). The feeding habits of the albacore in the Santa Marta coastal area were low in terms of richness of food items. There is no information on its feeding habits for the Colombian Caribbean. This research is the first approach to the trophic ecology of this tuna. Worldwide, this type of research on this species is scarce. In general, it has been shown that fish are the main and most diverse prey in its stomach content (Aloncle and Delaporte, 1973; Ortiz De Zarate, 1987); between 32 and 44 food items have been recorded in T. alalunga, out of which nine were fish species, with the most important prey being Engraulis japonicus in terms of weight.
Some feeding preferences were found for the different size classes of T. alalunga. This species showed the same preference for megalopa larvae in sizes between 30 and 60 cm. Megalopa larvae were the most representative prey in terms of abundance. The presence of crustacean larvae has also been reported, as in this study. These were the most numerous prey (25.9 %) in the stomach content of T. albacares (Potier et al., 2007). Fish are the main prey in most studies on tuna and other large pelagic species, as they provide greater nutritional enrichment (Potier et al., 2007; Consoli et al., 2008). It is necessary to highlight the importance of invertebrate larvae in the diet of these tunas; they are frequently found in great abundance, although their energy contribution is lower when compared to other organisms such as fish or Cephalopoda. This study reported crustacean larvae, such as those of Megalopa and Decapoda, as well as larvae of the family Penaeidae and the phylum Brachiopoda. These larvae have been reported in other studies on the trophic ecology of the albacore and other tunas, in some cases being the most important prey found in stomach contents (Potier et al., 2007; Consoli et al., 2008; Varela et al., 2017).
Engraulis japonicus has been reported as the main prey of the albacore, as well as the cephalopod Gonatopsis borealis, according to its RII (Ruiz-Pérez et al., 2016). The diet of the tunas showed significant differences over the studied period, and its prey composition and diversity was low. There was no evidence of trophic overlap between the two species, which is probably due to the fact that these species feed mainly on fish, but also on other prey such as megalopa and Stomatopoda larvae.
The low Levin and Shannon-Wienner indices indicate a limited trophic niche breadth and a low prey diversity. The dominant and most frequent prey in the stomach contents of these tuna suggest a preference, probably because they provide a higher percentage of energy to their diet. Still, the feeding strategy of the tunas is related to the abundance of prey in their ecosystem and their ability to select the most profitable prey (Consoli et al., 2008). In this research, prey abundance and availability in the ecosystem was not studied. Based on our results and other previous studies, the tunas’ preference for fish was evidenced (García and Posada, 2013).
CONCLUSIONS
The importance of small pelagic fish was evidenced, as Teleostei remains were found in most stomachs and contributed greatly to the diet of the studied organisms. In the climatic seasons analyzed, significant differences were evidenced regarding prey abundance in stomach contents. Finally, most of the tuna prey stemmed mainly from epipelagic habitats (Sardinella aurita, Mugil sp., and crustacean larvae). Despite this, E. alletteratus and T. alalunga were classified as generalist predators and with low prey diversity. Therefore, it is advisable to conduct trophic ecology research while following combined stomach content approaches and stable isotope analyses in order to more accurately determine the trophic behavior of these organisms.











 text in
text in