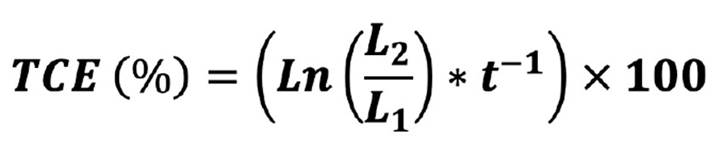INTRODUCTION
Rodophyta harbors approximately 7554 species and is regarded as one of the macroalgae phyla with the largest number of species, boasting a diversity of shapes and sizes (Mateo-Cid et al., 2020; Guiry, 2024). Within this group is Chondrachanthus chamissoi (C. Agardh) Kützing 1843, commonly known in Peru as yuyo and mococho, and in Chile as the sea chicorea (Riofrío, 2003; Macchiavello et al., 2012; Vidal and O’Ryan, 2015). It is found in rocky areas of the intertidal and subtidal zones, with a distribution that spans the western coast of South America, from Paita-Perú (5°S) to Ancud-Chile (42°S) (Ramírez and Santelices, 1991; Icochea, 2008), although it has also been recorded in the coasts of Korea, Japan, and France (Yang et al., 2015). This species features a membranous thallus ranging from 6 to 45 cm in height, with varying colors from dark green to reddish-brown or black. It has a flattened multiaxial thallus with distichous branching, producing primary lamellar axes with lateral branches that display either flat or cylindrical pinnules (Acleto, 1988; Arakaki et al., 2018). Chondracanthus chamissoi has an isomorphic and three-phase lifecycle, with alternating gametophytes (male and female), carposporophytes or cystocarpics (fertilized female individuals), and tetrasporophytes (Calderón et al., 2010, Ávila et al., 2011).
This species has been subject to the overexploitation of its natural populations (Carbajal et al., 2005; Pariona and Gil-Kodaka, 2011), given its use for direct human consumption and its carrageenan content (Acleto, 1986; Bulboa et al., 2013; Saavedra et al., 2019; Ávila-Peltroche and Padilla-Vallejos, 2020). As of 2006-2007, an increased extraction of this resource had been recorded in the algae’s natural meadows in the areas of Puerto Nuevo (35.6-89.9 g/m2) and Lobería (36.5-88.3 g/m2), both in the Pisco province of Peru. However, in 2010, a decrease in the collection of the average biomass of both localities was recorded (Flores et al., 2015). The current market demand for the species cannot be met with the current availability of the resource in natural populations (Flores et al., 2015). Therefore, the cultivation of C. chamissoi has been carried out in Chile and Peru. In Chile, suspended cultures are the main farming method, using fronds through vegetative propagation and spore culture or the inoculation of juveniles from spores (Macchiavello et al., 2003, 2007; Bulboa et al., 2005; Bulboa, 2006; Bulboa and Macchiavello, 2006; Cahui, 2018; Sáez and Macchiavello, 2018; Basaure et al., 2020), managing to incorporate these cultures into an integrated multi-trophic aquaculture (Koste, 2017). In Peru, studies have been conducted on vegetative propagation in different substrates (Pariona and Gil-Kodaka, 2011), spore-based cultivation (Arbaiza, 2016; Castañeda et al., 2017; Arbaiza et al., 2019), and the assessment of some factors involved in the growth rate (Riofrío, 2003). However, studies on the algae’s regenerative capacity are scarce (Acleto, 1986).
Regeneration involves a whole recovery process in the affected area (as a result of a necrosis) from nearby areas (cells), given that the damaged cells’ loss of cytoplasmic material creates a mucilaginous layer over the affected zone. Thus, when the fronds are cut, the algae can repair them. This ability depends on the species and the extent of the damage and comprises two stages: the restoration of the damaged area and the re-establishment of growth (Tornbom and Olivera, 1992; Buschmann et al., 1999; Ram et al., 2000). Multiple studies have determined the importance of algae regeneration in farming (Gómez and Westermeier, 1991; Echegaray and Seoane, 1992; Santelices and Varela, 1995; Correa et al., 1999; Scrosati, 1999; Sáez et al., 2015), mainly characterized by the presence of diffuse apical and intercalary meristems (Candia et al., 2006). Species such as Sargassum muticum, Gracilaria verrucosa, Iridaea laminaroides, Cystoseira mediterranea, Gracilariopsis heteroclada, Hizikia fusiformis, and Kappaphycus alvarezii have shown a response to the regeneration process (Fletcher and Fletcher, 1975; Kling and Bodard, 1987; Gómez and Westermeier, 1991; Echegaray and Seoane, 1992; Kyoung et al., 1999; Hurtado-Ponce, 2002; Hurtado and Biter, 2007; Yong et al., 2014). In the case of Laminaria hyperborea, the presence of phlorotannins seems to play an important role (Halm et al., 2011). Nevertheless, the regeneration rate of C. chamissoi in Peru has not been determined, and the culture yields for each farming system are not known, which is a fundamental factor for knowing the productivity and real performance of cultures in order to improve planning and production efficiency. Therefore, the objective of this work was to determine the regenerative capacity of the algae C. chamissoi in two cultivation systems (suspended and bottom) in the central-southern coast of Peru between August 2019 and February 2020.
MATERIALS AND METHODS
Study area
This study was conducted in the marine concession of the Algas Marinas Artisanal Fishery Workers Cooperative (Cotrapalmar), located in La Puntilla (13° 48’S, 76° 15’W), north of Paracas bay, in the district of the same name, in the province of Pisco, department of Ica (Figure 1). The concession is 200 m away from the coastline and has a maximum depth of 4 m, as well as a total area of 5 ha. This study was conducted during the 2019-2020 period, specifically between August 2019 and February 2020.
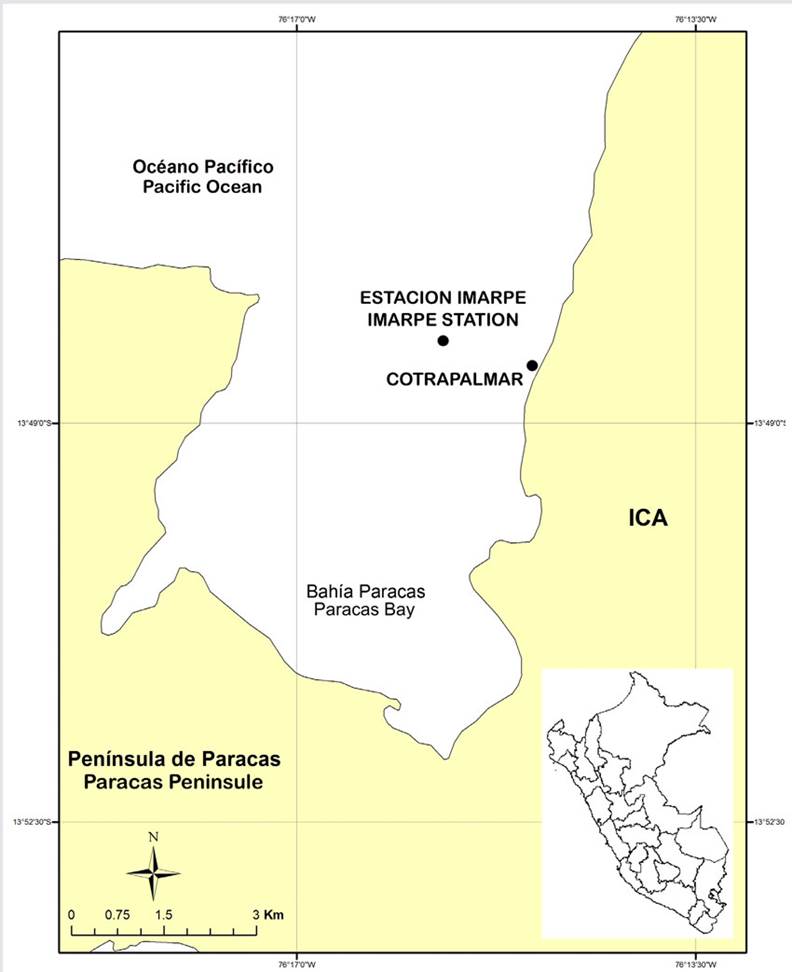
Figure 1. Location of the marine concession belonging to the Algas Marinas Artisanal Fishery Workers Cooperative (Cotrapalmar), La Puntilla, San Andrés, Pisco-Peru. The dots indicate the location of the marine concession and the physicochemical parameters sampling station of the Peruvian Institute of the Sea (Imarpe).
Physicochemical variables
The physicochemical variables were taken from the monitoring station of the Peruvian Institute of the Sea (13° 48’ 33” S-76° 15’ 45” W), which is very close to the study area. The variables measured for this study were temperature and salinity.
Obtaining cultivation units
The polypropylene strings inoculated with C. chamissoi individuals, regarded as sample units (SUs), were provided by the Marine Culture Research Laboratory (LICMA) of Universidad Científica del Sur, and they were the result of spore-based cultivation. The strings were sown in Cotrapalmar’s marine concession in January 2019, and their sea growth period lasted eight months before the first pruning (August 2019).
Cultivation systems
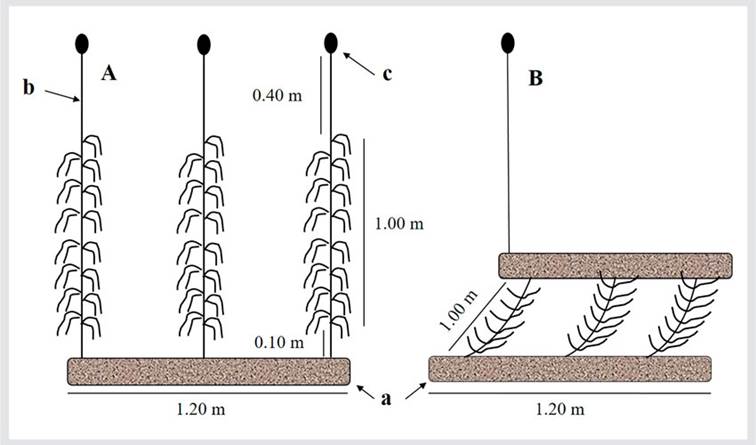
The creation of cultivation systems involved anchoring the SUs to sleeves made from fishing nets measuring 0.20 x 1.20 m, filled with rounded stones. For the suspended cultivation system (SC) (Figure 2A), three suspended systems were installed as replicas, each one with three SUs (n = 9), 0.3 m apart, covered with thalli of C. chamissoi 4-5 cm in length, and installed at 2-4 m deep. The SUs were vertically supported with the help of a buoy attached to the upper end of each string (Figure 2A).
For the bottom cultivation system (BC) (Figure 2B), three systems were also installed as replicas, at a depth of 4 m, with three SUs each (n = 9), covered with thalli of C. chamissoi 4-5 cm in length. In each replica, the strings were tied at their two ends to the sleeves, with a separation of 0.3 m between strings. They were maintained in a horizontal position and at 0.15 above the sea floor. A demarcating buoy was tied to each replica from the surface (Figure 2B). Prior to the start of the study, the systems were installed and monitored for 15 using strings with no inocula, in order to confirm their stability on the sea floor.
Evaluation of regenerative capacity
Collection and transport
Sampling was carried out via semi-autonomous diving and randomly, manually cutting the main axes and branches of the plants, taking care not to remove the basal disk, as indicated by Acleto (1986), and extracting all specimens longer than the string (‘linear meter’). Then, the SUs were marked for monitoring. We evaluated a total of 18 SUs per month.
An initial pruning of the SUs was carried out (August 2019, winter) to favor their first regeneration. Afterwards, two post-harvest prunings were performed in November 2019 (spring) and February 2020 (summer), cutting or extracting only the axes and main branches, leaving a 4-5 cm length of C. chamissoi on average. The biomass collected from each SU was placed in labeled Ziploc bags, which were separated by cultivation system and taken to the LICMA in a thermal container at a temperature no lower than 10 °C for characterization.
Pretreatment
In the LICMA, the samples were washed with seawater, extracting any trace and impurity that could affect the measurement of the wet biomass. Likewise, macroscopic separation by lifecycle phases was performed by differentiating fronds such as cystocarpic (C), tetrasphoric (T), and vegetative (V) plants (Acleto, 1986; Bulboa and Macchiavello, 2006).
Biometric variables
Monthly monitoring was carried out for in situ growth data collection, recording the length (cm) of all pruned individuals while performing maintenance tasks (cleaning of epibionts and sediments). After pruning (every three months), the length (cm) was recorded with the help of a vernier, and the biomass (g), as well as the accumulated biomass, was measured using a digital balance with a 0.01 g accuracy. This was done in the facilities of LICMA (ex situ), considering the phase of the thallus.
Evaluating the specific growth rate (SGR)
Using length data, the regenerative capacity was expressed by calculating the specific growth rate (SGR), taking the equation proposed by Orduña-Rojas and Robledo (1999) as reference:
Statistical analysis
The RStudio software was used. The length, biomass, SGR, and phase data were analyzed via normality (Jarque-Bera) and homoscedasticity (Bartlett) tests. For the biomass data, the T-Student test was carried out during the first harvest, and, for the length data, the Mann-Whitney U test was conducted. As for the second harvest, since the assumptions of normality and homoscedasticity were not fulfilled, the Mann-Whitney U test was performed. To analyze the morphology of C. chamissoi individuals based on length and biomass data, a two-way ANOVA was carried out for the data with a normal distribution, as well as an SRH test for non-parametric data.
RESULTS
Morphological characterization of the specimens
The characterization and identification of the development phase of the C. chamissoi plants was performed at the macroscopic level: the fronds with no reproductive structures were labeled as vegetative (V); those with the presence of cystocarps were labeled as carposporic (C); and those with the presence of tetrasporangial sori were labeled as tetrasporic (T). Carposporic and tetrasporic plants showed a wide/thick thallus, unlike the vegetative ones, with a prevalence of a brown-greenish coloration. Most specimens exhibited multiaxial thalli, producing more than two main axes with branches of different sizes (Figure 3).

Figure 3. Monitoring process for C. chamissoi: a: size at the beginning of the experiment after the initial pruning (August 2019); b: measurement during monitoring 1 (September 2019); c: measurement during monitoring 2 (October 2019); d: individual from the initial pruning (August 2019); e and f: individuals from post-harvest 1 (November 2019); g: individual from post-harvest 2 (February 2020). *CF: bottom cultivation, or BC; CS: suspended cultivation, or SC; C: carposporic frond; V: vegetative frond; T: tetrasporic frond.
Physicochemical parameters
Between the beginning of the experiment and the first post-harvest (winter-spring, August to November 2019), the average temperature was 17.5 °C, and the average salinity was 35.1. Until the second post-harvest (spring-summer, November 2019 to February 2020), the recorded average temperature was 20.5 °C, with an average salinity of 34.7.
Biometric variables and specific growth rate (SGR)
First post-harvest
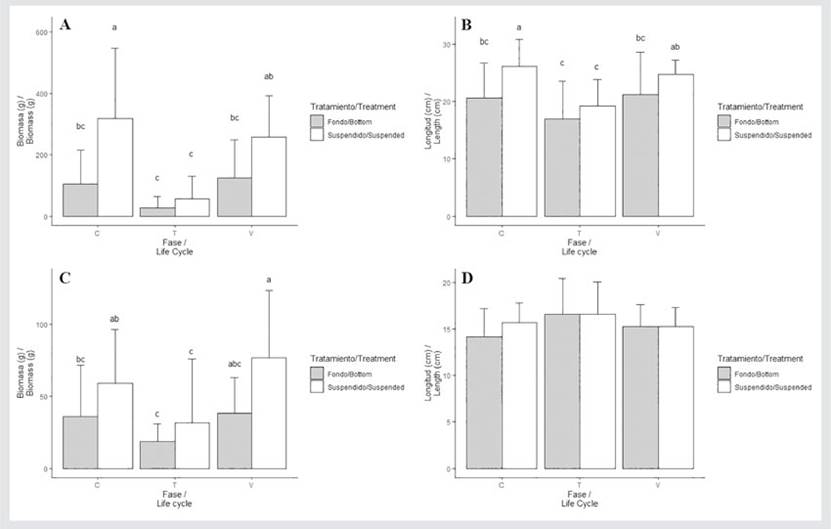
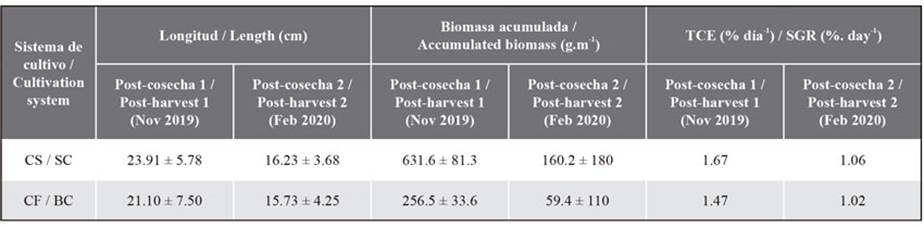
For BC, the initial biomass and length before the first post-harvest were 1.02 ± 0.87 g and 4.68 ± 1.01 cm, respectively, while, for SC, the reported values were 1.50 ± 0.67 g and 4.28 ± 1.07 cm, with no significant differences found (biomass: p = 0.09; length: p = 0.35). In the first SC post-harvest (spring 2019), 103 days after the initial pruning, it could be observed that C. chamissoi exhibited an average length of 23.91 ± 5.78 cm and an average accumulated biomass of 631.6 ± 81.3 g.m-1 (N = 157), finding significant differences (length: p = 3e-5; biomass: p = 0.003) with regard to BC, which reported an average length of 21.10 ± 7.50 cm and an average accumulated biomass of 256.5 ± 33.6 g m-1 (N = 128, Figure 4). Meanwhile, the SGR of the length was not significant for both treatments (p = 0.13).
Second post-harvest
The initial length for the second post-harvest were 5.44 ± 0.65 cm and 5.63 ± 0.75 cm for BC and SC, respectively. After 102 days, the second post-harvest was carried out (during the summer of 2020), and no significant differences between the two types of cultures were recorded (p = 0.25), although the frond length in SC was slightly greater (16.23 ± 3.68 cm) than that of BC (15.73 ± 4.25 cm). On the other hand, in the biomass analysis, significant differences were found (p = 0.046) in the final results for SC and BC, obtaining average biomass values of 160.2 ± 180 gm-1 and 59.4 ± 110 gm-1, respectively. Finally, like in the first post-harvest, the SGR showed no significant difference between cultures (p = 0.10) (Table 1) (Figure 5).
Table 1. Average length (cm), average accumulated biomass (g.m-1), and SGR (%. day-1) in suspended (SC) and bottom (BC) cultures. Post-harvest 1 was carried out 103 days after the initial pruning, while post-harvest 2 was conducted 102 days after the first harvest. Sample sizes: post-harvest 1: SC (n = 157), BC (n = 128); post-harvest 2: SC (n = 157), BC (n = 93).
Proportion of reproductive phases in the cultivation systems
When the first pruning was carried out (August 2019), the predominance of vegetative fronds was observed (55.52 % for BC and 50.20 % for SC), followed by carposporic fronds (BC = 34.55 %; SC = 38.44 %) and tetrasporic fronds (BC = 9.93 %; SC = 11.36 %) (Table 2). The first post-harvest (November 2019) for BC showed a predominance of vegetative fronds, followed by carposporic plants and, finally, tetrasporic plants. Meanwhile, for SC, the following was observed: carposporic phase -> vegetative thalli -> tetrasporic phase. Regarding biomass (ANOVA) and length (SHR and Multiple Comparisons), significant differences between cultivation systems (ANOVA: p = 0.001; SHR: p = 0.01) and lifecycles (ANOVA: p = 0.0006; SHR: p = 0.02) were found (Fig. 6A and 6B) (Tables 2 and 3).
Table 2. Proportion for each lifecycle of C. chamissoi, calculated based on biomass. V: vegetative frond; C: carposporophyte; T: tetrasporophyte.
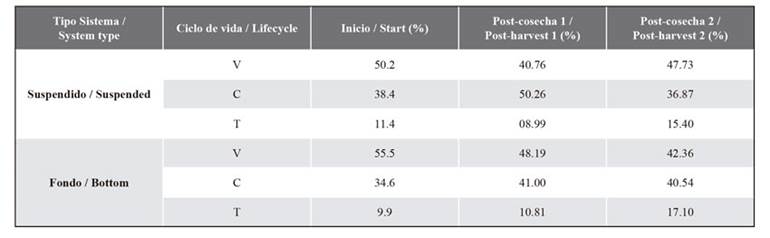
Table 3. Biomass (g.m-1) for each lifecycle of C. chamissoi. V: vegetative fronds; C: carposporophyte; T: tetrasporophyte.
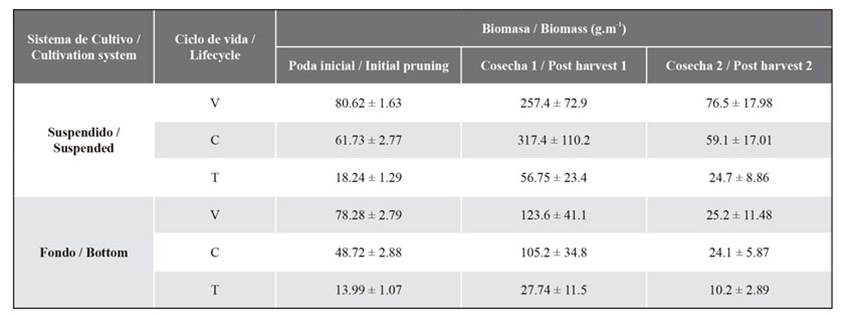
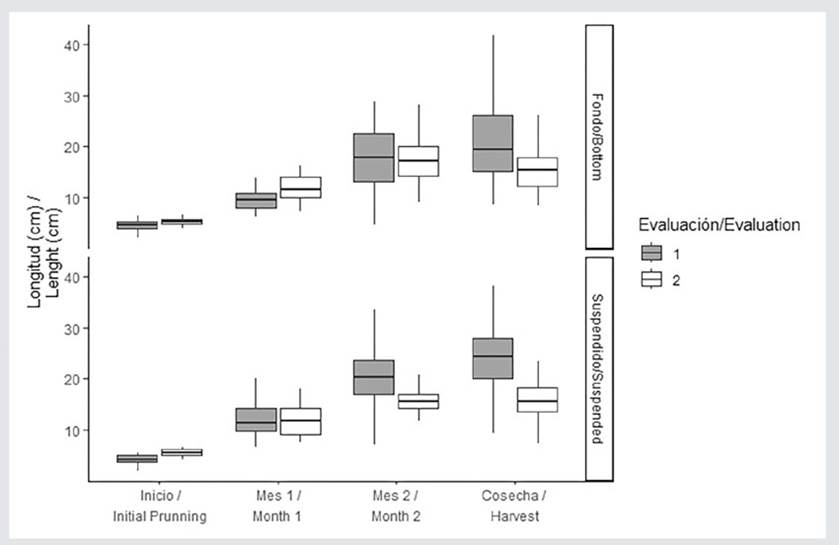
Figure 5. Length (cm) of C. chamissoi during the first and second harvest. Evaluation 1 (dark boxes) corresponds to the time period from the initial pruning to harvest 1, while evaluation 2 (white boxes) corresponds to the time period between harvests 1 and 2. The start corresponds to the initial pruning (evaluation 1, August 2019) and harvest 1 (evaluation 2, November 2019). Month 1 and month 2 correspond to the months of monitoring: September and October 2019 for evaluation 1 and December 2019 and January 2020 for evaluation 2.
During the second harvest (February 2020), a similar trend was observed in BC and SC: there is a predominance of vegetative fronds, followed by the carposporic and tetrasporic phases. The SRH and Multiple Comparisons test performed for the biomass showed significant differences (ANOVA: p = 0.03; SHR: p = 0.01) between cultivation systems (p = 0.03), but not between lifecycles (p = 0.06). Meanwhile, the two-way ANOVA found no significant differences for length with respect to the cultivation system (p = 0.54) or the lifecycle phases (p = 0.33) (Figures 6C and 6D) (Table 2 and 3).
DISCUSSION
The SUs underwent monthly monitoring until the third month. At this time, in the first post-harvest, an average algae length of 21.10 ± 7.50 cm was recorded for BC, as well as 23.91 ± 5. 78 cm for SC. These results are similar to those obtained by the Acuisur S.A.C. company in San Juan de Marcona (average length of 22.5 cm), albeit after five months of cultivation (Zapata, 2018). On the other hand, Cahui (2018) recorded average growths of 17-18 cm after 65 days in SC in Peru’s southern coast. These values are similar to those obtained in this work for the second harvest (16.23 ± 3.68 cm on average for SC in three months).
One of the advantages of algae which facilitate their cultivation is the lack of tissue differentiation, facilitating vegetative propagation, in addition to the malleability of their reproduction cycles and the variability of their metabolism (Machado, 2015). In the case of C. chamissoi, the advantage is its fast healing process, which prevents infestation by contaminants or pathogens that may delay regeneration (Sáez et al., 2015). Moreover, its recovery times are dependent on the extent of the damage (Mooney and Staden, 1985; Sáez et al., 2015), the age of the algae, and the presence of antioxidant compounds such as phlorotannins (Halm et al. 2011). Thus, the use of juvenile specimens is recommended (Fletcher and Fletcher, 1975). The specimens used in this work had seen no previous harvests, which favored their growth. In the case of other algae such as Fucus sp., the regeneration of adventitious branches on the surface of the wound is a slow process (Fulcher and McCully, 1969) that depends on the distance to the vegetative apex. Therefore, it is better to perform cuts near the apex. In the case of Agarophyton chilense (ex Gracilaria chilensis), no significant differences were found between the length of the fragments cut with or without apical tips during regeneration processes (Santelices and Varela, 1995). On the other hand, Correa et al. (1999) managed to improve the regeneration time of fragments (from the center and the edge of the algae) of Gigartina skottsbergii, with a temperature of 15 °C and an irradiance of 5 µmol m-2s-1, as well in cut hapteria. Likewise, in Gracilariopsis lemaneiformis, regeneration takes place from the main branch, which grows while forming secondary branches. These branches, after later cuts, may assume the regenerative capacity of a main branch (Zhou et al., 2016). Therefore, each algae species has its own characteristic regeneration mechanism.
The cell wall of C. chamissoi consists of two layers: an internal, solid layer and an external one (gelatinous). Both layers are composed of carbohydrate polymers (Riofrío, 2003). As mentioned by Acleto (1986) , a manual extraction or pruning of the longest axes or branches allows the shorter axes to be responsible for maintaining growth and/or favor regeneration (Sáez et al., 2015). Kling and Bodard (1987) indicate that the regeneration process of Gracilaria verrucosa depends on the cells below the affected area, with cortical cells (Collantes et al., 2004) being responsible for fulfilling this function. The pruning to which this species was subjected took place every three months, and, as mentioned by Gómez and Westermeier (1991), performing few intensive prunings is not advisable, as the algae’s energy level decreases, which translates into slow healing processes and increasingly prolonged frond regeneration times.
The factors affecting the regeneration process are diverse: from the type of cut to the harvesting method, physicochemical factors, seasonality, and epiphytism, among others (Acleto, 1986; Gómez and Westermeier, 1991; Echegaray and Seoane, 1992; Lobban et al., 1994; Correa et al., 1999; Mantri et al., 2011; Macchiavello et al., 2017). In light of this, it is assumed that human manipulation may play an important role, as an intense extraction often generates a biomass reduction in the area, which in turn affects the extractor’s economy. The exposure of the algae to areas of low depth and close to the coast (Arbaiza et al., 2019) affected our work by reducing the size and biomass in post-harvest 2 for both cultivation systems.
SC turned out to perform better than BC in terms of biomass and length, with results similar to those of other red algae species such as Kappaphycus alvarezii and Eucheuma denticulatum (Kimathi et al., 2018). On the other hand, the better performance in the strings of SC may also be due to the fact that they were in closer contact with sunlight, which favors the growth and development of C. chamissoi (Hurd et al., 2014) thanks to its direct relationship with photosynthesis processes, considering the photoperiod and the irradiance (Macchiavello et al., 2017). This is demonstrated by the greater length and biomass occurring at lower depths in this study (Table 1). Likewise, Bulboa and Macchiavello (2001) determined the direct relationship between the growth rate (GR) of C. chamissoi and temperature increases, achieving the best GR (8-16 %/day) at a temperature of 25 °C. However, Pacheco-Ruíz et al. (2005) mentioned that fronds of C. squarrulosus show rapid aging with increased temperature and irradiance.
Another study conducted by Macchiavello et al. (2003) records the production of spores in the species throughout the year, with a considerable peak in spring and a lower one in summer. This is strictly related to the increase in biomass in the cystocarpic phase and then in juvenile vegetative individuals, which is very similar to what occurred in this study, where the biomass gain of post-harvest 1 (November 2019, spring) was higher for BC and SC than that obtained in post-harvest 2 (February 2020, summer) (Table 3).
In works carried out by Universidad Católica del Norte with strings from spore culture, biomass values between 217 and 300 g.m-1 were obtained in intervals of 2-4 months at 3 m deep. In addition, similar studies on the vegetative cultivation of C. chamissoi have reported results of 435 g.m-1 in three months at depths of 3 m. These results are superior to those of harvest 1 in BC (256 ± 33.6 g.m-1) but inferior to those of SC (631 ± 81.3 g.m-1), which may be related to the amount of light to which SC is exposed, as it is at lower depths (Saavedra et al., 2019).
CONCLUSIONS
It was evidenced that C. chamissoi can regenerate and reach its extraction size in approximately two months when manual pruning is performed, leaving 4-5 cm on average to facilitate regeneration. In addition, suspended cultivation systems exhibit a better performance in terms of biomass and length. Nevertheless, regenerative capacity decreases with each pruning, which is why sowing new seeds after three harvests is recommended.











 text in
text in