Introduction
The genetic diversity of cacao and differences in its biochemical characteristics generate a wide range of fermentation conditions. This is an important stage in the cacao postharvest, where a series of biochemical transformations induced by microbial action (microbial succession) develop, modifying the substrate in the pulp into substances of interest that modify the sensory profile of cocoa beans.
The cacao microbiota, which has been shown to intervene positively during fermentation, is composed of yeasts, lactic acid bacteria (LAB), and acetic acid bacteria (AAB) 1. When the fermentation stage is prolonged for more than four days, bacilli, enterobacteria, and filamentous fungi, desired or not, can also participate in the cocoa fermentation process 2. Yeasts have been related to the production of volatile organic compounds generators of typical aromas of some fermented foods, associated with the production of endogenous enzymes that induce the generation of chemical precursors of flavor such as free amino acids, peptides, and reducing sugars 3-5.
The application of starter cultures in fermentation has been studied to improve microbial dynamics and biochemical transformations in the process, reduce time, and improve the sensory quality of the bean. Recently, interest has grown in using yeasts as starter cultures for cocoa fermentation, especially species of the Saccharomyces, Pichia, Kluyveromyces, Candida, and Torulaspora genera 6,7. Furthermore, some studies evaluated the application of mixed starters composed of yeast and LAB 5,8. The great capacity to assimilate citric acid, reducing sugars, pectinolytic activity, resistance to stress conditions, production of volatile compounds, and antagonistic capacity against pathogens has directed research towards using these microorganisms as a biotechnological strategy to promote sensory quality in cocoa 9,10. This requires the selection of microorganisms resistant to the conditions of the fermentation process, guaranteeing a high transformation of substrates and generating products of interest that modify the sensorial quality of cocoa to show a greater aroma or flavor. For this reason, there is interest in isolating promising microorganisms to be inoculated during the cocoa fermentation phase. This research aims to select yeasts according to their morphological characterization, behavior during the fermentation process, and the production of aromas through a sensory evaluation, with potential application for optimizing the fermentation phase and sensory quality of cocoa.
Methodology
Microorganisms
Eight yeasts were evaluated, six isolated from fermentative masses of cocoa-producing farms in the departments of Huila, Tolima, and Antioquia, Colombia, including Hanseniaspora opuntiae (Y01), Saccharomyces cerevisiae (Y02), Pichia kudriavzevii (Y03), Debaryomyces hansenii (Y04), Aureobasidium pullulans (Y07), and Wickerhamomyces anomalus (Y08) of the microorganisms working collection of Agrosavia with interest in nutrition and animal health. The other two, ATCC 36633 Candida versatilis (Etchells and Bell) Meyer and Yarrow (Y05) and ATCC 28253 Zygosaccharomyces rouxii (Boutroux) Milenrama (Y06), were certified commercial yeast strains selected for their production of aromatic compounds.
Yeast tolerance tests to simulated cocoa fermentation process conditions
The microorganisms were subjected to resistance tests, inoculating 1,000 µL of the microorganism suspension with turbidity equal to McFarland tube 3 in 9,000 µL of water peptone at 0.1 % in 20 mL test tubes. Prior to inoculation, the malt extract broths were conditioned at soluble solids concentrations of 10, 15, and 20 °Brix, ethyl alcohol concentrations at 5 and 10 %, acetic acid with pH of 3, 4, 5, 6, and 7, and temperatures of 30, 40, and 50 oC in the broths. The cultures were incubated for 48 h under constant agitation at 120 rpm, quantifying viable cells in decimal dilutions 10-4, 10-5, and 10-6 at the end of the incubation, according to the methodology proposed by Jurado Gámez et al. 11 and Lozano Tovar et al. 12. The uninoculated culture medium was used as a control.
Substrate utilization test
The yeasts were inoculated individually in 300 mL of sterile cocoa pulp medium with a 3:1 pulp:water ratio, adding 15 mL of suspension of the microorganism with turbidity equal to McFarland tube 3 and incubating under constant agitation at 1,200 rpm and temperature of 30 °C for 35 h, using the adapted methodology of Briceño Vélez 13. The fermentations were carried out in triplicate, taking aliquots at 0, 5, 10, 15, 20, 25, 30, and 35 h of fermentation, quantifying the content of soluble solids, reducing sugars, pH, titratable acidity, percentage of ethyl alcohol and viable cells, following the methodologies detailed as follows.
Soluble solids, ethyl alcohol, pH, and titratable acidity: The aliquots at different fermentation times were analyzed for total soluble solids in °Brix, taking 1 mL of the sample and measuring in a portable refractometer PAL-1 (3810) (Atago Co. Ltd, Tokyo, Japan). The percentage of ethyl alcohol was registered with a digital refractometer PAL-34S (4434) (Atago Co. Ltd, Tokyo, Japan) in percentage (%). The volatile acidity or pH in the sample was quantified in an aliquot of 10 mL using a potentiometer (Thermo Scientific). Total acidity was estimated in percentage (%) by reducing the acidity present in the sample with a basic 0.1 N NaOH solution 14.
Reducing sugars: Dilutions of 1:1000 v/v were made to quantify fermentable sugars in the cocoa pulp medium. All samples were homogenized for 5 min and passed through a 0.22 µm millipore filter. The content of reducing sugars was calculated using the 3,5 dinitro salicylic acid (DNS) method, a compound that, in the presence of heat, is reduced by the reducing sugars present, developing a color change. It was measured in g/L using a spectrophotometer SQ2800 Unico at a wavelength of 575 nm, estimating the reducing units present in sugars based on a glucose standard curve 15.
Viable cells: The viability or viable microorganisms count was estimated by diluting 1 mL of the aliquot at different fermentation times in 9 mL of 0.01% peptone water. Decimal dilutions were made up to 10-6; the last three dilutions were cultured in malt extract medium placed in Petri dishes and in superficial extension with glass beads. The dishes were incubated at 35°C for 48 h, counting the colonies on the plate, which were multiplied by the dilution factor and by 10, expressing the results in colony-forming units per milliliter (CFU/mL) 16.
Sensory analysis to detect aroma production in cocoa pulp
In 150 mL of cocoa pulp medium in a 3:1 pulp:water ratio, 15 mL of microorganism suspension was inoculated at turbidity equal to McFarland tube 3, incubating at low oxygen pressures for 48 h, in triplicate. The 20 mL aliquots were evaluated by trained tasters using the descriptive olfactory profile method. Samples were analyzed in terms of odor descriptors (sour, sweet, floral, fruity, alcohol, herbal, and yeasty), using an ordinal scale from 0 to 10, where 0 means absence, 1 to 2 low intensity, 3 to 5 medium intensity, 6 to 8 high intensity, and 9 to 10 very high intensity. The panel was also asked about their three preferred samples concerning aroma formation 17.
Statistical analysis
The data on resistance to yeast fermentation dynamics and substrate utilization were normalized. Subsequently, parametric analysis of variance (ANOVA) tests were performed, followed by the multiple comparison test for differences between the Tukey means with a 95% confidence level using the RStudio statistical software.
Results and discussion
Tolerance to different pH conditions
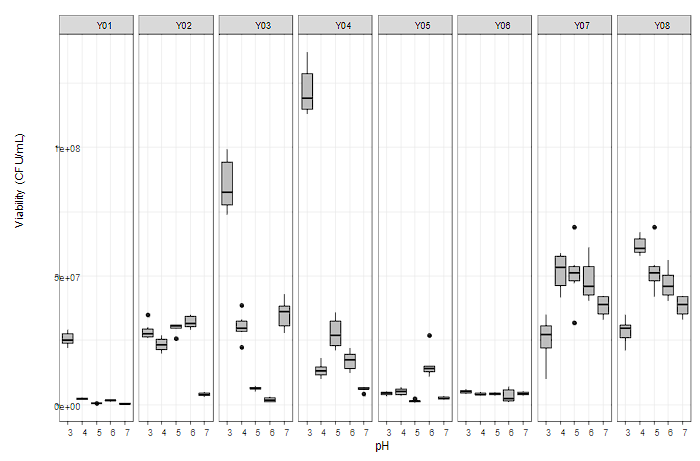
Cocoa fermentation shows a microbiological dynamic that generates biochemical changes in parameters such as pH. Figure 1 shows the tolerance expressed in the viability of the yeasts studied when subjected to different pH conditions, taking as reference what was expressed by authors such as Bilenler et al. 18, who reported as the minimum required viability for microbiology|1ical starters applied in foods a value of 106 CFU/g.
The yeast Hanseniaspora opuntiae Y01 reported the highest viability at a pH of 3, which was drastically reduced when subjected to other pH values of the biochemical dynamics, reaching values below 6 log at pH values of 5 and 7. This behavior is analogous to the cocoa microbial diversity study results, where this species was isolated only at the beginning of fermentations 10, and its detection was not possible at later fermentation times.
The viability of S. cerevisiae Y02, Z. rouxii Y06, and A. pullulans Y07 was only affected when subjected to pH values of 7, 6, and 3, respectively, without registering statistically significant differences in the other pH values studied. Yeast W. anomalus Y08 did not report statistically significant differences in pH, maintaining its viability among the highest microbiological density levels compared to the other yeasts in the study. In this way, its growth capacity at different pH conditions and adaptability to different biochemical conditions were demonstrated, positioning it as a potential yeast to be incorporated into biochemically dynamic fermentative processes without its viability being affected 19.
Tolerance to different temperature conditions
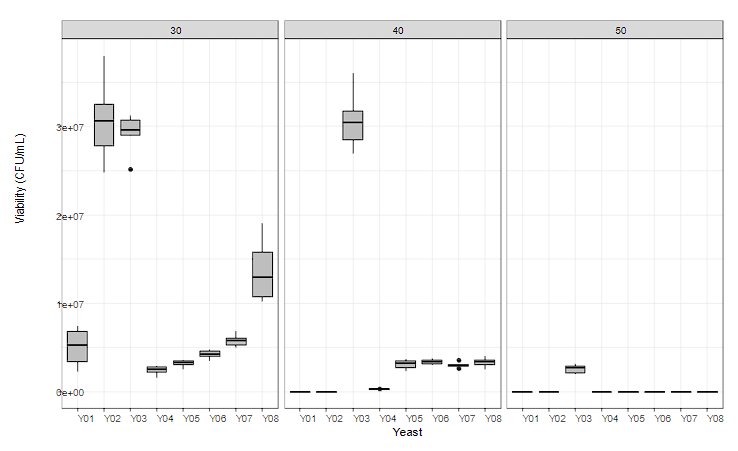
Figure 2 shows the tolerance of the yeasts under study to different temperature conditions of cocoa fermentation dynamics. Yeasts must be resistant to temperatures above 45 °C to guarantee the death of the seed embryo, so this factor is decisive in the selection of the microorganism 20,21.
The high-temperature tolerance of the yeast P. kudriavzevii (Y03) was highlighted, being the only viable strain exposed to temperature conditions of 50 °C, maintaining its viability above 6 Log, coinciding with the report of its effectiveness in growth capacity and high-temperature ethyl alcohol production from sugar cane juice, corn stalk hydrolyzate and cassava hydrolyzate 22. Additionally, this species recorded the highest viability at all the temperatures studied. Strains D. hansenii (Y04), Z. rouxii (Y06), A. pullulans (Y07), and W. anomalus (Y08) recorded statistically significant differences at a confidence level P > 0.05 for the temperature factor being its viability affected by the thermal level 23
Tolerance to different soluble solids conditions
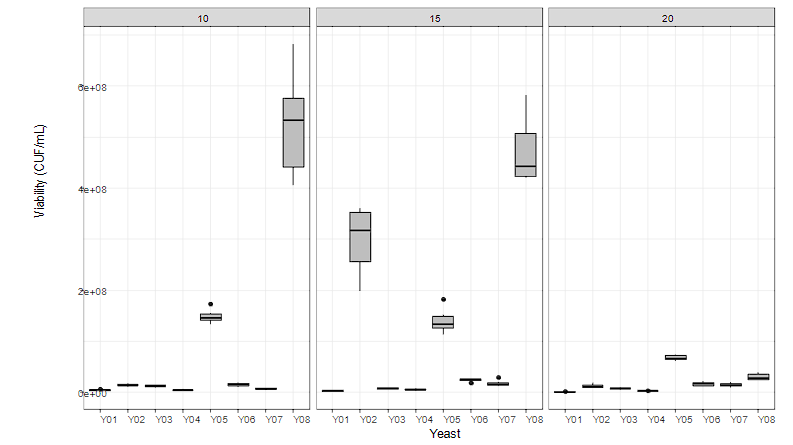
The genetic variability of cacao materials and their bromatological composition demand a starter culture for cocoa fermentation, with the capacity to tolerate differences in the soluble solids content of its pulp without affecting its viability. The results of the tolerance test of the studied yeasts are recorded in Figure 3. Strain W. anomalus (Y08) registered the highest viability at 10 and 15 °Brix, with 8 log and 7 log in culture media with soluble solids contents of 20 °Brix (Figure 3). Its osmotolerance was highlighted, also recorded by the strain Candida versatilis (Y05), which has been isolated from apiaries and used in soybean fermentation processes, emphasizing its capacity to convert sugars into ethyl alcohol, organic acids metabolism, and volatile compounds formation, describing it as a tolerant and adaptive yeast.
Tolerance to different ethyl alcohol conditions
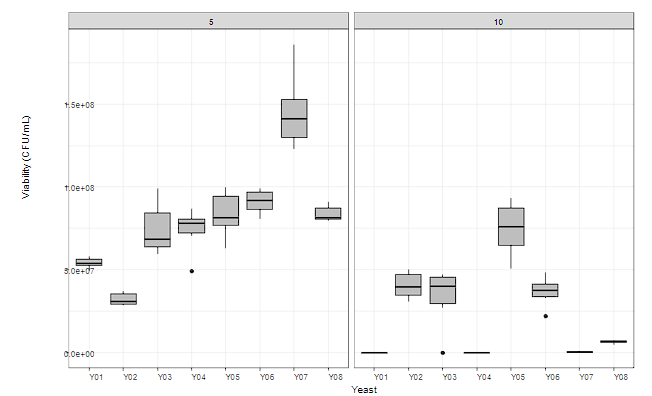
Figure 4 shows the tolerance of the yeasts under study to 5 and 10 % ethyl alcohol concentrations.
No statistically significant differences were recorded in the viability of the yeasts when they were exposed to 5% ethyl alcohol. However, with the increase in ethyl alcohol concentration, yeast strains such as Y01, Y04, and Y07 were strongly affected in their viability. The resistance of strains Y05, Y03, Y02, and Y06 to high concentrations of ethyl alcohol in the culture was highlighted, registering viabilities above 7 log, which, in strains such as P. kudriavzevii (Y03), demonstrated its ability to withstand adverse conditions during cocoa fermentation by remaining viable under conditions of high ethyl alcohol concentration and temperature. Therefore, it can be considered a promising microorganism to be used as a starter culture in cocoa fermentation.
The tolerance tests allowed generating a first screening of the yeasts studied to select a starter culture to improve the microbial dynamics during fermentation and the sensory quality of cocoa beans. The yeast strain H. opuntiae (Y01) was discarded as it registered low tolerance in factors such as pH, soluble solids content, temperature, and ethyl alcohol concentration of 10%, reflected in its low viability and resistance to the biochemical dynamics of cocoa fermentation.
Substrate utilization consumption test
The capacity and speed of transforming macromolecules into a substance of interest are other selection parameters that the starter culture must meet. Yeasts interact in the first phase of cocoa bean fermentation under acidic conditions and low oxygen pressures, generating various transformations, as stated by Delgado-Ospina et al. 24. Ethyl alcohol is produced from citric acid and carbohydrates (sucrose, glucose, and fructose), present in the mucilage of ripe cacao fruits, which partially diffuses into the cotyledons of the cacao bean, oxidizing later on due to the effect of acetic acid bacteria 3,25. Figure 5 shows the viability, substrate utilization capacity, and acid and alcohol production of the yeasts studied. Yeast strains Y02 and Y03 showed the culmination of their exponential phase at 10 h of fermentation in cocoa pulp medium, which for strains Y07 and Y08 was reported at 25 h of fermentation. Yeast Zygosaccharomyces rouxii (Y06) recorded a prolonged stationary phase by maintaining its highest viability between 15 and 25 h of fermentation. These results show the specificity in the viable behavior of yeasts of different genera on the same substrate 23.
The content of soluble solids showed a decreasing behavior for the five preselected yeasts in the 35 h of fermentation, with the highest decrease found in the musts of Z. rouxii (Y06) and S. cerevisiae (Y02). A similar evolutionary profile was recorded in the content of reducing sugars generated by the utilization of carbohydrates and glucose, which were hydrolyzed from the sucrose of cacao pulp by invertase activity. This explains the increase reported in the reducing sugar content of W. anomalus (Y08), P. kudriavzevii (Y03), and S. cerevisiae (Y02). During the first five hours of fermentation, they showed a greater availability of fermentable sugars or monosaccharides that are subsequently transformed into ethyl alcohol as a primary product and carbon dioxide and glycerol as secondary products 10,25.
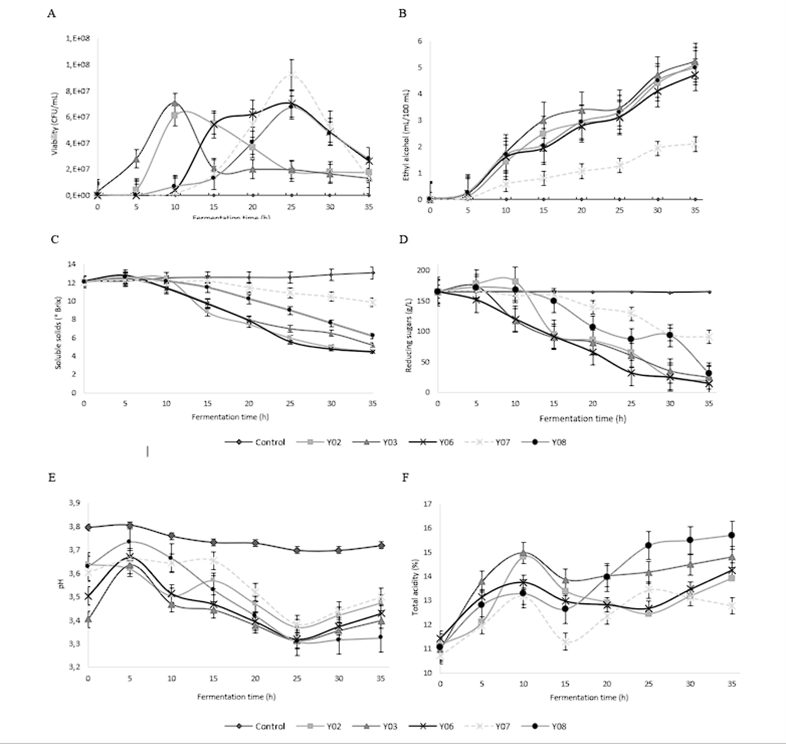
Figure 5 Substrate utilization dynamics by preselected yeasts. Viability (A), Ethylic alcohol production (B), Soluble solids dynamics (C), Reducing sugars (D), Volatile acidity (E), and Titratable acidity (F).
The pH presented a decreasing behavior during the first 25 h of fermentation with the five preselected yeasts, which demonstrates the heterofermentative capacity of the yeasts under study, capable of producing organic acids such as acetic acid and succinic acid 26,27, compounds probably responsible for the increase in the total acidity of the medium. After 25 h of fermentation, an increase in pH was recorded, possibly generated by reducing the main carbon source (carbohydrates) and promoting the consumption of acids in the medium.
No statistically significant differences were recorded in the ethyl alcohol production of P. kudriavzevii Y03, W. anomalus Y08, S. cerevisiae Y02, and Z. rouxii Y06. However, P. kudriavzevii Y03 and W. anomalus Y08 were those with the highest production of the metabolite of interest, recording a lower consumption of reducing sugars and total acidity, evidencing a higher capacity to transform sugars and acids present in the cacao pulp into alcohol, required for the death of the embryo and subsequent diffusion in cacao beans during the aromatic development of the beans 6,20
Production of yeast aromas in cacao pulp musts
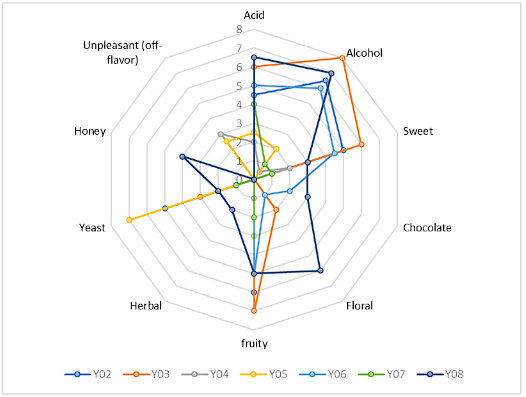
The production of aromas during cacao fermentation has been mainly associated with the microbiological consortium of yeasts in the process 10,25,28,29. It is subjected to factors such as the nutrient concentration of the substrate, biochemical conditions of the process, and yeast genetics that, in turn, intervene in the production of enzymes and generation of metabolites of interest, as expressed by Kongor et al. 30 and Moreira et al. 31. The aroma production test in the cacao pulp substrate of the yeasts under study is shown in Figure 6 through the aroma profile of the musts.
The qualification of fine and aromatic cocoa requires the presence of specific notes in its sensory profile, highlighting yeasts such as P. kudriavzevii Y03, S. cerevisiae Y02, Z. rouxii Y06, and W. anomalus Y08, which recorded the highest intensity in the fruit aroma of the musts studied with average values of 7, 6, 5 and 5, respectively. The floral aroma was detected in greater intensity in the aromatic production of yeast Z. rouxii Y08, a species that also recorded herbal and honey notes in its profile that were not found in the fermented musts of the other yeasts studied, agreeing with the high production of ethyl acetate reported for this species and that has been related to fruity notes 32. Phenylethyl alcohol and 3-methyl-1-butanol acetate detected in rice wine fermentation are associated with fruity and floral notes; hence, this species stands out for its aromatic production 33. The presence of alcohol was detected at a higher intensity in the fermented musts of yeasts P. kudriavzevii Y03, Z. rouxii Y08, and S. cerevisiae Y02. Since this compound is required for the death of the embryo during cocoa fermentation, its greater presence can favor the release of compounds that act as precursors of organoleptic molecules. Yeasts like D. hansenii Y04 and C. versatilisY05 recorded in their profile unpleasant aromas (off-flavors) in intensities of 3 and 2.5, respectively, which were described as overfermentation or decomposed fruit. These, together with the low production of specific aromas and alcohol and less resistance to biochemical dynamics compared to the other yeasts studied, allow their exclusion from the yeast selection process as a starter culture for cocoa fermentation.
Conclusions
Yeasts W. anomalus (Y08) and P. kudriavzevii (Y03) were selected for their ability to transform substrate into products of interest during the fermentation of cacao pulp, with the production of specific aromas and resistance to adverse biochemical conditions typical of cacao fermentation. The selected microorganisms have a high feasibility of application as a fermentation promoter starter culture that can be used individually, by consortium, and as a mixed culture.