INTRODUCTION
Amphibians are an important component of the biota present in many ecosystems, and in some cases are considered the largest fraction of vertebrate biomass, thus contributing to the trophic dynamics of the communities to which they belong (Valencia-Aguilar et al. 2013). However, the loss, fragmentation and transformation of natural habitats as a consequence of human activities (Knutson et al. 1999) in the last 50 years has generated a considerable and irreversible loss of diversity in ecosystems (Andrade-C., 2011). It is believed that there are approximately 277 species of anurans nationwide in some category of threat (Acosta Galvis, 2019).
The family Centrolenidae groups the commonly known glass frogs due to its translucent skin, distributed in 10 genera and 79 species approximately for Colombia (Acosta Galvis, 2019). This group of amphibians is well represented in the humid forests of the Pacific (Bustamante et al. 2007; Rengifo & Lynch, 2010). The central area of the department of Chocó has a registry of six genera and approximately 13 species.
The ecological contribution of amphibians to ecosystems, together with the global disappearance of a large number of species and the decline of 43% of their populations worldwide (Stuart et al. 2004; Wake & Vredenburg, 2008), reflects the urgent need to carry out immediate actions that deepen in the knowledge of the specific causes of decline, in the study of their biology, ecology and in the design of conservation strategies.
The Centrolenidae family presents a variety of characteristics and behaviors that make it possessor of a high species richness, for this reason, it is important to understand the ecology and distribution in the forests of the department of Chocó, where some studies by Hayes & Starrett (1980), Lynch & Suárez-Mayorga (2004) and Rengifo & Lynch (2010) have shown that the central area of the department of Chocó tropical rainforest presents a high potential to house a high richness of species of centrolénidos, however, the change of land use and the transformation of habitats at the hand of man (Gibbs et al. 2010; Carr, 2004) could be affecting the diversity of organisms due to the reduction of plant cover.
In the department of Chocó, the diversity of amphibians is very important, however, many species lack the assessment of their conservation status, particularly in areas where diverse anthropogenic actions exert a negative pressure, which could lead to the reduction of their diversity (Myers et al. 2000), which is a cause for concern because many of the species of the Centrolenidae family are not very tolerant of the transformation of their habitats and require bodies of water with favorable conditions for their development. The present study aims to generate information about the structure and habitat use of species of the family Centrolenidae in an area currently impacted by different anthropic activities, which will allow the development of future management and conservation plans with the communities that live in the areas of Tropical rainforest.
MATERIALS AND METHODS
Study area. The study area was located between the geographic coordinates of 5º00'-6º45'N and 76º30'-77º15'O (Table 1). The precipitation regime is bimodal-tetra-stational with a period of higher rainfall between April and October and a period of lower concentration from November to March (Poveda et al. 2004). The annual rainfall is on average 8558mm with a monthly average of 395.5mm (Rangel-Ch. & Lowy, 1993). The general climatic limits are an average temperature higher than 24°C (Table 1).
Table 1 Location and biophysical characteristics of the five sampling areas in a tropical rain forest in the central area of the department of Chocó. Temperature (Temp °C), Relative humidity (%), Average annual precipitation (PP mm), Tropical rain forest (bpt). Data were taken from Rengifo et al. (2014).

The five sampling zones, Pan-American Union, Certegui, Lloró, Atrato, and Quibdó, are located in the alluvial plains, low hills and foothills in the central area of the Chocó department of tropical rainforests (bp-T), where the largest concentration pluviosity of the Pacific platform is concentrated (Chocó Biogeográfico). Although the forests of the Chocó Biogeográfico are homogeneous, these zones present a great variety of ecosystems, which means the presence of suitable conditions to show a wealth of remarkable fauna and flora, however, the region is being affected by some actions anthropic, such as mining, selective extraction of fauna and flora and plantation of agricultural crops (Palacios Rodríguez et al. 2018; Rengifo M. et al. 2014; Rengifo M. et al. 2019).
Fieldwork. The present study was conducted in periods between March and June 2016, with a duration of six days in each vegetation cover. Two types of vegetation cover (primary and secondary forest) were selected and replications were made at the five sampling points. It is considered the primary forest, such as that which has existed without significant human disturbance and other disturbances during periods exceeding the normal life span of mature trees of 60 to 80 years according to FAO (ANON, 1982). The secondary forest, corresponds to a stage of the forest cover that arises after a total anthropogenic intervention (of more than 90%) of the primary vegetation, creating regeneration conditions, which lead to a structure different from the original forest, with another composition of arboreal species and another dynamic, without having yet reached its original state again, that is, it is clearly differentiated from the state of the original forest (ECO, 2000).
In the sampling sites, the aforementioned plant coverings were qualitatively identified. For the primary forest, observations were made around trees, shrubs bordering the current water currents of waters, rocks, soil. For the secondary forest, places with little vegetation, bodies of water with apparently low quality or with some type of contamination (transformed habitats) were observed.
The samplings were carried out at night times from 6-10pm. through free and unrestricted travels through surveys by Visual Encounter Survey (VES) (Crump & Scott, 1994), using a sampling effort of 240 hours/person (120 hours Primary Forest and 120 hours Secondary Forest).
The identification of the registered species was made based on specialized guides (Renjifo & Lundberg, 1999; Páez et al. 2002; Ron et al. 2018) and scientific articles (Guayasamín et al. 2009; Ruiz-Carranza & Lynch, 1991a; b; Ruiz-Carranza & Lynch, 1998). Additionally, the determinations were corroborated with reference material from the Herpetology Collection of the Technological University of Chocó. Additionally, a bibliographic review of the centrolénidos species registered for the area under study was carried out.
During the samplings, records of some ecological data suggested by Heyer et al. (1994) as the vertical position or height of the perch (from the ground or water) and classified in the following ranges: soil (0cm), low (1 - 49cm), medium (50 - 149cm), high (> 149cm). The type of substrate or perch site was classified as branches, stems, leaves, litter, rock. The information on the state of conservation and endemism was documented according to the IUCN (2018).
Data analysis. To determine if the sampling effort in the study areas was sufficient to know the species richness, non-parametric estimators such as CHAO2 and ICE were used, which relates the accumulated species according to the sampling effort, using presence and absence data of registered species using the EstimateS Version 6.0 program. The alpha diversity was evaluated by determining the number of individuals in each cover and the relative abundance was determined by dividing the number of individuals per species in each cover, over the total of captured individuals, and also the capture success was calculated to determine the representativeness of the applied sampling effort.
In addition, the dominance index (Simpson) and equity (Shannon-Wienner & Pielou) were used (Baev & Penev, 1995). Beta diversity, defined as the similarity of species among the sampling areas (primary forest and secondary forest), was analyzed using the Jaccard similarity coefficient (Magurrán, 1988).
Nonparametric methods were used to compare the coverage and determine if there are significant differences in the composition and use of habitat through the PAST program. Version 1.15 (Hammer & Harper, 2003). X² chi-square was performed to evaluate habitat use.
RESULTS AND DISCUSSION
Composition of Species. In total, 53 individuals belonging to six species and four genera were recorded (Table 2, Figure 1), in a sampling effort of 240 hours/person, obtaining a capture success of 0.22 individual/hour.person. The bibliographical review carried out in this geographical area reports the presence of five species of centrolénidos that were not registered in this study (Cochranella ramirezi, Espadarana prosoblepon, Hyalinobatrachium chirripoi, Sachatamia albomaculata and Sachatamia ilex) event that could be related to the different anthropic interventions that currently facing these ecosystems due to habitat removal due to mining at different scales, the indiscriminate felling of trees, and contamination of water bodies by contamination with chemicals, which are the main habitats of this faunal group, possibly generating the decrease in the populations of this group of amphibians.
Table 2 Taxonomic composition, total (n) and relative abundance (%) of centrolénidos in the vegetal coverings sampled in the department of Chocó. Primary forest (BP), secondary forest (BS), number of individuals (N), relative abundance (%).
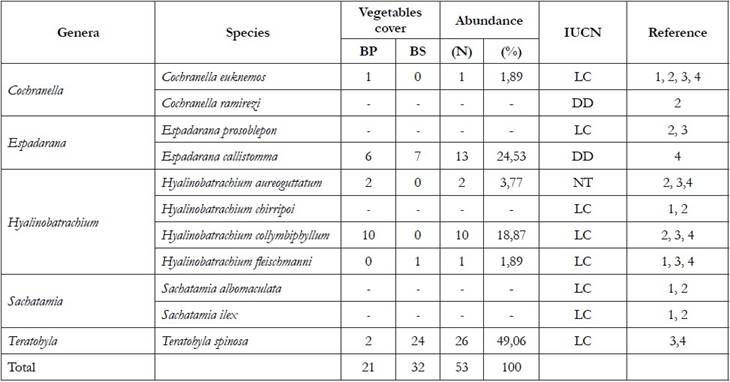
LC: Least Concern; DD: Deficient Data 1) Hayes & Starrett, 1980; 2) Lynch & Suárez-Mayorga, 2004; 3) Rengifo & Lynch, 2010; 4) Registered in the present study.

Figure 1 Species of centrolenids present in areas of tropical rain forest in the center of the department of Chocó. a. Cochranella eucknemos; b. Espadarana callistomma; c. Hyalinobatrachium aureoguttatum; d. Hyalinobatrachium collymbiphyllum; e. Hyalinobatrachium fleischmanni; f. Teratohyla spinosa.
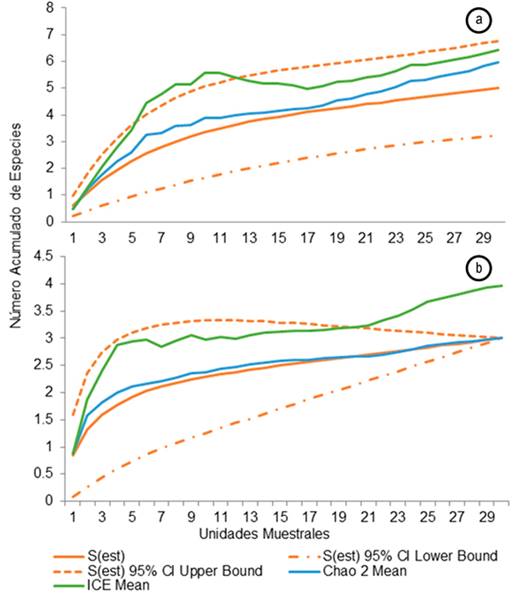
The non-parametric wealth estimators in the two vegetation cover sampled showed different behaviors; for the primary forest, the Chao2 estimator indicates that the registered species equals 83.3% while for ICE 76.1% of the estimated species (Figure 2a). In the secondary forest area, the registered species are equivalent to 75% of the species estimated by ICE, while according to Chao2, the registered species correspond to the totality of the species with the effort of inverted sampling (Figure 2b). When estimating the richness of centrolénidos for the primary forest and to graph the curve of accumulation of species, this did not reach its asymptote, indicating that some additional species could be registered when intensifying the effort of sampling. For the secondary forest, on the other hand, the species accumulation curve reaches its stability, showing that the sampling effort applied to this area was significant since the species estimated for this coverage were recorded. The behavior of the curve of the primary forest is normal for the tropical ecosystems since the prolonged work and in different periods of the year are those that achieve the stabilization of the curves (Vargas & Bolaños, 1999; Rengifo M. et al. 2015). that it is necessary to carry out samplings with more time to register more species that could possibly be found in these sites and be able to evaluate the status and size of their populations, the possible threats they may be facing and factors that could be having a negative impact.
Of the four registered genera, Hyalinobatrachium was the best represented with three species, while the rest of genera were represented by a single species, data that coincide with a study conducted by Vargas & Bolaños (1999) in an inventory of frogs in the bass Anchicayá, Valle del Cauca, finding the genus Hyalinobatrachium as the best represented within the family. Of the total of recorded species T. spinosa was the most abundant species with 49% (n = 26) and the rest of the species were represented together with a percentage of 50.9% (Table 2). In this study we recorded the species T. spinosa, H. fleischmanni and E. callistomma in secondary forest areas, the fact that these species are found in this type of habitat could indicate that some species of centrolénidos present resilience or tolerance to modifications in their habitats causing these species to persist in these ecosystems, the secondary forests without direct and obvious intervention in the surrounding vegetation of the streams, could present the conditions that allow the reproduction and development of some species since the presence of some species of centrolénidos it is determined by the males when selecting the sites with the ideal conditions for their reproductive processes (Rojas-Morales & Escobar-Lasso, 2013), Guayasamín et al. (2009), affirm that centrolénidos species require streams with permanent flow and the presence of suspended vegetation to adhere their mass of eggs. As has been demonstrated in some investigations with glass frogs (Ramírez J. et al. 2009; Basto-Riascos et al. 2017), however, it is important to use conservation strategies since some species of centrolenids do not tolerate changes or transformations in their habitats, which evidences an imminent risk for these species.
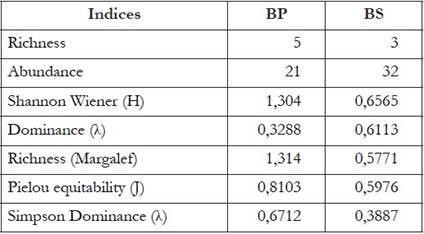
According to the analysis of nonparametric estimators, no significant difference was found between the coverages evaluated with respect to the composition and use of habitat (P = 0.6759), which may be related to the closeness and forestry continuity of both coverages, making both forest types (primary and secondary) are similar, which shows that the support vegetation in both forests is significant, which allows the presence of these species in these sites. The Jaccard similarity coefficient reveals that the primary forest zone shares 33.3% of the species with the secondary forest, among which are E. callistomma and T. spinosa. The dominance-diversity index indicates that H. colymbiphyllum was the most abundant species for the primary forest and T. spinosa for the secondary forest. Some species vocalize from the high perches, mostly from the beam or the back of the leaves to attract the females for the amplexus and they register in this type of heights to avoid being easily preyed by crawling animals, due to greater availability of food and favorable conditions. for the development of their populations (Cardozo-Urdaneta & Señaris, 2012) (Table 3).
Table 3 Wealth and diversity values of Cristal frogs for the sampled coverages (primary forest and secondary forest). BP = Primary Forest, BS = Secondary Forest.
Regarding the registered hanger height for each of the species, there was no significant statistical difference (X2 = 7.12, P = 0.21) within the evaluated heights, but when analyzing the percentage values a greater number of records on high hangers, with 73.5%, followed by middle positions with 26.4%. Cochranella euknemos is the species recorded at the highest level of vegetation structure in the primary forest, followed by the species T. spinosa (Figure 1f). For the secondary forest, H. fleischmanni was the highest recorded species with only one individual, followed by the E. callistomma species (Table 4). The species T. spinosa presented a higher number of individuals in high and medium positions, followed by the E. callistomma species in both coverages while H. colymbiphyllum was recorded in this type of height only in the primary forest, the results agree with (Rada et al. 2007; Cardozo-Urdaneta & Señaris, 2012), where they refer that centrolénidos species were registered in high and medium positions.
Table 4 Vertical position and substrate of the glass frogs in the study area. (N = number of individuals). Number of individuals (N), relative abundance (%).

All the individuals of centrolénidos were registered to heights greater than 100cm of the ground, as they refer different studies carried out (Ortega-Andrade et al. 2013; Rojas-Morales & Escobar-Lasso, 2013; Vargas S. & Castro H., 1999), show that the species H. aureoguttatum was registered in terms of its vertical location or height in medium and high position, data that agree with the results of our investigation. Although few species were recorded in the secondary forest area, all were above 2m, this may be because this coverage presents traces of the natural forest, which makes it contain trees, shrubs, and good vegetation, allowing to find these species in this type of coverage and also, some species prefer the perches to ensure the reproductive success of their eggs and to avoid crawling predators or being trodden by other animals that use these sites as forages (Rueda-A., 1994).
There was no significant statistical difference (X2 = 4.71, P = 0.45) in terms of substrates or perch sites (leaves and branches) where the family species Centrolenidae were recorded (Table 4), but by percentage comparison, the leaves were the substrate with the largest number of records with 83% and the branches with 17%. The species T. spinosa, E. callistomma and H. colymbiphyllum presented a greater number of individuals in the leaves showing a greater preference for this substrate, which could be due to the fact that the leaves are more preferred sites where the centrolénidos perform the amplexus, the so-called or singing of the males, parental care and the deposition or postures of their eggs so that they fall directly into the water and complete their development cycle (Rojas-Morales et al. 2011; Ortega-Andrade et al. 2013).
According to the IUCN (2018), the species H. fleischmanni, H. collymbiphyllum, T. spinosa, and C. euknemos are in a state of “Least Concern” (LC) however E. callistomma is in the category of "Deficient Data"(DD), and H. aureoguttatum is registered as an" Almost Threatened "species, which shows the importance of conserving the habitats of these species, since their low representativeness or abundance could indicate that the anthropic actions would be affecting populations of these species.