Introduction
Pathologies caused by parasites are considered to be one of the most devastating and prevalent infections worldwide, causing morbidity in millions of animals in the livestock industry in endemic areas (Momčilovićet al., 2019). Gastrointestinal parasites (GIPs) are therefore regarded as one of the most important health problems in cattle, as they cause economic losses in livestock productions at a global level and generate adverse effects on their sanitary status (Pinilla et al., 2018). In general, gastrointestinal parasitism in cattle is caused by protozoa and helminths (nematodes, trematodes, and cestodes) and, most of the time, its manifestation is multi-etiological (Craig, 2018).
The clinical signs prevalent during parasitism may vary and depend on the parasite load, the species involved, and the individual's immunity. The most common signs include a decreased weight gain rate, diarrhea, rough coat, weakness, and anemia. Other symptoms may also be observed depending on the organ involved. In addition, parasitic infections commonly predispose hosts to various diseases, especially in subclinical or long-standing cases (Bowman, 2014; Craig, 2018; Healey et al., 2018). Economic calculations have been made which only consider production reductions, without accounting for the financial impact associated with veterinary fees and the cost of field personnel, thus determining that economic losses due to gastrointestinal nematodes are approximately 445 million dollars per year (Rodríguez-Vivas et al., 2017).
Thanks to its geographical location, Colombia has an extensive altitudinal range, which results in different environmental conditions that favor the development of the biological cycles of a great variety of GIPs in cattle. However, in the department of Boyacá, few studies report the prevalence of said parasites (Pinilla et al., 2019). The objective of this research was to identify the main families of GIPs present in cattle in the central province of the department of Boyacá.
Methodology
Geographical location
The study included the municipalities that are part of the central province of the department of Boyacá, which constitutes 70% of its territory. This area is characterized by containing the most significant proportion of the population and municipalities of the department. Its climates are temperate, cold, and paramount, with temperatures between 11,4 and 15 °C, altitudes ranging from 2.480 to 2.908 meters above sea level (masl), and a relative humidity between 82 and 89%. It is a smallholder area with political and industrial power (Estupiñán-Pedraza, 2014).
Sample size
According to the National Livestock Census conducted by the Colombian Agricultural Institute (ICA, 2018), 156.690 cattle were reported in the central province of the department of Boyacá. Based on this information, a sample of 716 individuals was calculated with 95% confidence, using the statistical program OpenEpi (version 3) with the following formula, as expressed by Equation (1):
where:
Sample collection and processing
65 herds were sampled, with an average of 15 to 20 cattle. 716 fecal samples (approximately 25 g) of female and male dairy cattle of the Ayrshire, Jersey, Holstein, and Normando breeds and different age groups were collected by rectal palpation using the gloved hand technique. The samples were labeled with the number of the experimental unit, stored in ICOPOR coolers at 4 °C, and transported to the Veterinary Parasitology Laboratory of Universidad Pedagógica y Tecnológica de Colombia (UPTC) for a coprology test.
The samples underwent double-blind testing, and they were processed using the formalin-ether or modified Ritchie method to identify eggs, larvae, and cysts. Stool samples were sedimented by centrifugation in an ether-formaldehyde system, as described by Uttaro et al. (2011).
Variables
Before sampling, an epidemiological survey was conducted, which evaluated the age, breed, sex, diarrhea, respiratory symptoms, and deworming variables. Among the possible risk factors associated with the farms, the presence of a management pen, drinking water sources (aqueduct, cistern, and stream), and the type of feed offered (silage, hay, and concentrate) were also assessed.
Statistical analysis
A descriptive cross-sectional study with simple random sampling was performed. The association between the variables of the epidemiological survey and the prevalence was evaluated using the Chi-square test of independence, where the reference categories were an age group of 2-3 years and the Ayrshire breed, whereas, for the management practices, the absence of deworming and the other variables constituted the reference categories. The variables that showed a significant statistical association were analyzed by logistic regression using the IBM SPSS Statistics 19 software.
Results
The general prevalence obtained was 95,6% (685/716), which indicates that some stage of the GIP families was identified in the evaluated samples. Likewise, the descriptive analysis allowed determining that the prevalence for each family was distributed as follows: 53,8% for the Trichostrongylidae family (385/716), 47,1% for Eimeriidae (337/716), 10,5% for Taeniidae (75/716), 6,7% for Trichuridae (48/716), 5,3% for Strongylidae (38/716), 2% for Ascaridae (14/716), 2% for Strongyloididae (14/716), and 0,6% for Chabertiidae (4/716).
A significant statistical association was found between the breed of the evaluated individuals and the parasitic families Trichostrongylidae (p=0,000), Eimeriidae (p=0,000), Strongylidae (p=0,008), Chabertiidae (p=0,002), and Taeniidae (p=0,001) (Table 1). The age of the cattle showed a significant statistical relationship with the Strongyloididae family (p=0,00) (Table 2). Likewise, it was possible to establish that the manifestation of respiratory symptoms was related to the Trichostrongylidae family (p=0,002), while the presence of diarrhea was associated with the Strongylidae family (p=0,003) (Table 3).
Table 1 Breed as a risk factor associated with gastrointestinal infections caused by GIPs. The results are presented as Odds Ratio (OR) and at a 95% confidence interval (CI).
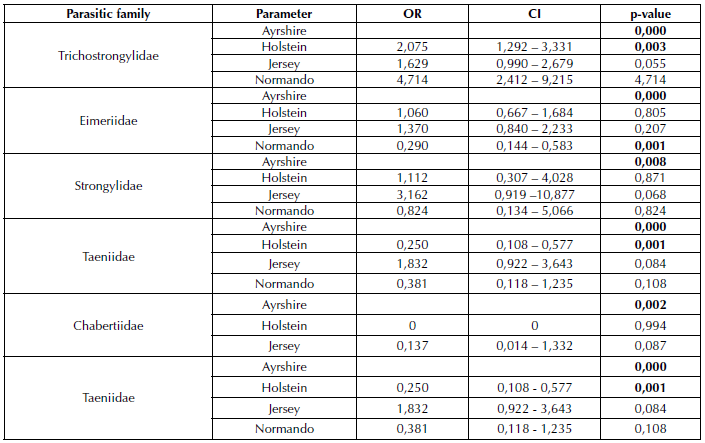
Table 2 Age as a risk factor associated with gastrointestinal infections caused by GIPs. Results are presented as OR and at a 95% CI.

Table 3 Variables as risk factors associated with gastrointestinal infections caused by GIP. Results are presented as OR and at a 95% CI.
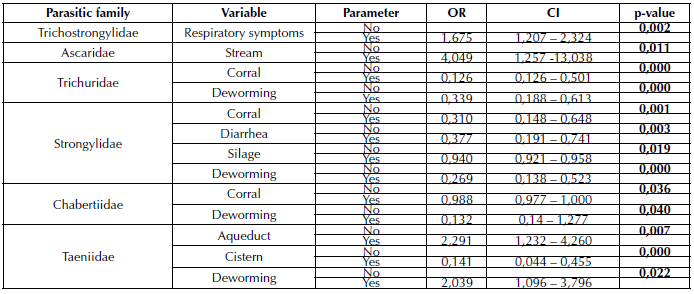
The analysis of variables such as sanitary management through deworming of the evaluated individuals showed a significant statistical association with the parasitic families Trichuridae, Strongylidae, Chabertiidae, and Taeniidae (p=0,000, p=0,000, p=0,040, and p=0,022, respectively), as well as a relationship between the implementation of the corral for cattle management and the presence of parasites of the Chabertiidae (p=0,036), Strongylidae (p=0,001), and Trichuridae families (p=0,000) (Table 3).
As for the water sources used in the productions, streams, aqueducts, and cisterns were found to have an association with Ascaridae and Taeniidae (p <0,05), whereas supplementation with silage was significant for Strongylidae (p <0,05).
Discussion
In Colombia, GIP prevalence values of 50,l5% (Pinilla-León et al., 2019) and 83,2% (Pinilla et al., 2018) have been reported, the latter being the closest to that reported in the central province of Boyacá. Likewise, Pinilla et al. (2019) determined a prevalence of 59% in Belén and 52,1% in Duitama (Boyacá), which is lower than the values obtained in this research. As for Latin America, in two districts of the Mantaro Valley (Perú), values of 24,5 and 30,3% have been reported (Briones-Montero et al., 2020), as well as a 39% prevalence in México (Fernández-Figueroa et al., 2015), in addition 47,8% for seasons with high rainfall and 46,2% for dry periods (Figueroa-Antonio et al., 2018). Prevalence values of 67,5% have also been reported in Perú (Colina et al., 2013), as well as 34,2% for Venezuela (Urdaneta-Fernández et al., 2011), with these being lower than those of this study.
Other regions of the world have reported prevalence values such as 51,5% in Pakistan (Shah et al., 2021), 60,46% in Iraq (Aram, 2020), 39,8% in Kombolcha city, Ethiopia (Ayele et al., 2020), 90,8% in Ghana (Squirere et al., 2019), 20,9% in Canada (Scott et al., 2019), 20,03% in India (Das et al. 2018), 40,6% in Germany (May et al., 2017), 38,8% in Indonesia (Nurtjahyani and Agustin, 2015), 86,9% in Taiwan (Huang et al., 2014), and 20,4 and 94,5% in Poland (Piekarska et al., 2013).
Among the possible factors that influence the variation of prevalence values are the high anthelmintic resistance developed by ruminants due to indiscriminate application of common antiparasitics (Geurden et al., 2014; Borges et al., 2015), climate factors that affect parasite biological cycles (Okulewicz, 2017), seasonal conditions that modify the intensity of parasite loads (Squire et al.,2019), and pre-seasonal rainfalls during the sampling phase that allow parasite development and dietary changes (Mahmood et al., 2014). Additionally, it should be considered that appropriate levels of humidity and temperature are required for the development of etiological agents and infective stages (Taylor et al., 2016), conditions that are more likely to occur in non-stabled farms (Squire et al., 2019).
The most prevalent families in cattle were Trichostrongylidae, Eimeriidae, and Taeniidae. However, in Venezuela, it was reported that the most frequent genera belong to the families Trichostrongylidae, Ancylostomatidae, and Strongylidae (Morales et al., 2012). Likewise, Sun et al. (2018) reported that the most prevalent parasites were Haemonchus, Ostertagia and Trichostrongylus, belonging to the Trichostrongylidae family, as well as Oesophagostomum of the Strongyloidae family. This variation may be due to environmental factors, the reproductive stage and sex of the animal, and the grazing and agricultural practices in farms. These factors play a determining role in the presence of infectious stages, thus favoring the development of reproductive cycles and the viability of eggs and larvae, which depend on the time of year, age, and immunological status of the host (Colina et al., 2013).
The age of the animals can also influence the parasite load, with the Strongyloididae family being the only one that showed statistical significance with this variable; individuals between 3 and 4 years old were less likely to get infected by parasites of the aforementioned family than bovines between 2 and 3 years old. This may be due to the fact that young cattle are susceptible to parasites, as demonstrated in previous research (Urdaneta-Fernández et al., 2011; Taylor et al., 2016; Briones-Montero et al., 2020). Therefore, keeping young animals in the same housing as older animals may expose them to parasitic infections (Pinilla et al., 2018; Squire et al., 2019). However, individuals in younger age groups may become more resistant to primary infection with some parasites as they reach maturity (Taylor et al., 2016).
Concerning the breed, contact with Holstein acts as a risk factor for infection by the Trichostrongylidae and Taeniidae families for the Ayrshire breed, which could mean that the former is more likely to suffer from parasitosis of these families than the Ayrshire breed cattle. However, the low amount of studies on parasites in dairy cows in tropical areas and their relationship with the breed of the animal hinders comparisons with the results obtained in this research. Da Silva et al. (2008) state that Holstein breed females, given their higher milk production, have a higher egg count per gram of feces than lower-production animals.
Since the response of ruminants depends on the nutritional status and metabolic requirements for milk production, there is a negative energy balance at the beginning of lactation. Therefore, there is a decrease in the expression of cytokines associated with the phenomenon of anthelmintic resistance (Pryce et al., 2000). Thus, specialized breeds (e.g., Holstein and Jersey) are more prone to suffer from GIPs because of their high production and their susceptibility to the stress of tropical climates, which decrease their immunological status, not to mention the management system used for the different breeds, which also causes stress (Silva et al., 2008).
The presence of in-farm pens acts as a protective factor against Trichuridae, Strongylidae, and Chabertiidae. Zootechnical factors play a significant role in the behavior of the incidence and intensity of parasitic invasion. The characteristics of the facilities and the area destined for the livestock farm, the type and form of feeding, the rearing system, and hygienic measures have a decisive influence on the conformation of the parasitological landscape of any herd (Soca et al., 2005).
Additionally, it should also be noted that deworming cattle is regarded as a protective factor against the presence of parasites of the Trichuridae, Strongylidae and Chabertiidae families, i.e., it is less likely that cattle will present these parasites, although, in the case of the Taeniidae family, deworming practices in production are considered to be a risk factor due to a condition that contradicts the biological relationship that may exist between the observed variables and the parasite family. Anthelminthic treatment is regarded as fundamental for helminth control in cattle given its ease of use, its relatively low cost, and the lack of effective alternative options (Woodgate et al., 2017). However, it should be taken into account that integrated parasite control entails harmony with helminths without them causing clinical conditions, as well as management strategies such as pasture rotation and selection of resistant and resilient animals, thus making it a more effective method to reduce parasitic infection levels (Bennema et al., 2010).
Regarding the water sources used for cattle, there is a negative relationship between streams and the Ascaridae family, probably as a risk factor, whereas, for the Taeniidae family, the presence of cisterns behaves as a protective factor, which does not agree with what was reported by Cornejo-Soto (2019), who found higher parasitic prevalence values in animals whose water consumption was supplied directly from ditches without receiving adequate potability treatment. Contaminated water is considered to be the main vehicle involved in the transmission of parasites. When ditches are used as a drinking water source, the possibility of GIPs in cattle increases, contrary to what occurs with an aqueduct system due to the physical-chemical processes performed to ensure optimal conditions for consumption (Cornejo-Soto, 2019).
Likewise, feeding practices contribute significantly to parasitosis, given that the oral route is the main access path for infective stages in the organism. Forages can constitute a source of infestation if they come from areas that have been fertilized with excreta or residues contaminated by infective stages of parasites (Soca et al., 2005; Henriques et al., 2021). This agrees with Urdaneta-Fernández et al. (2011), who state that the consumption of forage increases the risk of transmission of gastrointestinal nematodes, given that it is the main route of infection for most of the parasites that inhabit the intestinal tract. Thus, the consumption of silage acts as a protective factor against GIPs of the Strongylidae family since, in this case, feeding is not based solely on forage. In addition, the nutritional status of cattle can influence the severity of parasitic infection (Huang et al., 2014).
Conclusions
The results obtained in this research show the high presence of GIP in cattle of the central province of the department of Boyacá (95,6%), where the most prevalent families were Trichostrongylidae, Eimeriidae, Taeniidae, and Trichuridae. Likewise, age showed a significant statistical association with the Strongyloididae parasitic family. The breed variable of the individuals evaluated was associated with the Trichostrongylidae, Eimeriidae, Strongylidae, Chabertiidae, and Taeniidae families. The possible association of risk factors is related to the close relationship between the individuals and the parasitic families evaluated.















