Tamarind (Tamarindus indica L.) is an African native species (El-Siddig et al., 2006) adapted to semi- tropical arid conditions (Gupta et al. 2017). It is a very important crop for municipalities of the nearby Western of Antioquia (Colombia), since its fruit is offered in various presentations to the tourists who visit this region. Several local familiar confectionery microenterprises sell tamarind as a fresh fruit, or process the fruit to elaborate, pulps and juices. Antioquia is one of the two Colombian departments with the greatest tamarind production according to El Ministerio de Agricultura y Desarrollo Rural de Colombia - MADR (2013a), with the 30.3% of participation in the national production of this crop. In this department, the tamarind production is carried out in two municipalities of the nearby Western of Antioquia: Santa Fe de Antioquia and Sopetran, each of them with 59.9% and 40.1% of participation, respectively. The tamarind yield in the region of Antioquia (3.2 t ha-1) is lower than in the Atlántico region (5.6 t ha-1) (MADR, 2013b), this situation had to be analyzed with the purpose to improve the production of this fruit crop.
The tamarind fruit in the nearby western of Antioquia is obtained from dispersed trees or from traditional production systems. This fruit has low quality related to lightweight, small size, presence of insects and fungi that can discredit the products of small-scale confectionery enterprises (Correa, 2015). The most important pests of the fruit in the region, according to Muñoz and Rueda (2009) are Cadra cautella Walker, (Lepidoptera: Pyralidae) (or Ephestia cautella), Caryedon serratus Ol., (Coleoptera: Bruchidae) and Sitophilus linearis Herbst, (Coleoptera: Curculionidae). These insects cause high loss of fruits and low quality of the products elaborated with them, since, occasionally, insect parts are found in tamarind sweets (Muñoz and Rueda, 2009). In Mexico, the borer C. serratus is the biggest causative of damage, nevertheless, there are others Lepidoptera and Coleoptera that affect the fruit (Orozco et al., 2012): the spittlebug (Cercopidae), ants Atta and Acromyrmex and, the stem borer Trachyderes mandibularis Dupont, (Coleoptera: Cerambycidae) (Orozco, 2001; Orozco et al., 2009; Orozco et al., 2011).
Tamarind insect species of the most economic importance in India in the scale are Aonidiella orientalis Newstead, (Hemiptera: Diaspididae) (Patel, 2015), the mealybug Nipaecoccus viridis Newstead, (Hemiptera: Pseudococcidae) (Kumar, 2016) and, the fruit moth Tophlebia ombrodelta Lower, (Lepidoptera: Tortricidae) (Gupta et al., 2017). Other less important phytophagous species are Aspidiotus destructor Signoret, Planococcus lilacinus Cockerell and, Otinotus oneratus Walker (Butani, 1978; Ojo and Omoloye, 2015). Reports from other regions of the world show that tamarind is affected by different moths such as Mussidia nigrivenella Ragonot, Ectomyelois ceratoniae Zell., Plodia interpunctella Hübner, Cadra figulilella Gregson, Phidotricha erigens Ragonot (Solis, 1999), Paralipsa gularis Zell. (Kumar, 2016) and, Corcyra cephalonica Station (Devi, 2016).
The objective of this study was to determinate the phytophagous insects of the tamarind crop, focusing on those that cause the greatest fruit damage, in five farms in Santa Fe de Antioquia and Sopetran.
MATERIALS AND METHODS
Location
The study was carried out in five farms, four in the municipality of Sopetrán (6°30'21,5''N, 75°44'24,8''W) and one in the municipality of Santa Fe de Antioquia (6°33'18,35''N, 75°49,32''W) (Figure 1). These farms are located in a tropical dry forest zone (Holdridge, 1982) where the average temperature is 27 °C, the annual mean rain precipitation of 1097 mm and, the mean relative humidity is of 73.2% (Álvarez et al., 2015).
Sampling
In each farm, six sweet tamarind trees and six acid tamarind trees were selected, giving a total of thirty trees of each phenotypes, considered in such a way, because the characters that define a phenotype correspond in their great majority to the morphological description of the plant and its architecture (Álvarez, 2016). The trees of each phenotype were selected according to the criteria of each producer, corroborating the taste of the fruit. These were distributed in paddocks and their ages varied from 20 to 70 years of age.
A detailed revision of each tree was made in situ, as Nicholls (2008) suggests, from September to October 2015 and from January to February 2016 each 15 days. In addition, a sampling of the soil was made consisting in taking five subsamples from holes 20 cm deep distributed in the diameter of the area over the ground below the tree canopy, these samples were taken with a shovel.
The leaves and flowers that showed some symptom of damage were cut and stored in zip-ploc plastic bags, and the collection of insects from the symptom was done with a jama or wet brush. The adult insects were deposited in Falcon and Eppendorf tubes with 70% of alcohol with its data collection, to be identified later. The bark was revised in a similar way as the previous organs, from the base of the stem up to 1.8 m of high. In addition, a sampling of the soil around the tree was made, from which five samples were taken from under the diameter of the canopy of the tree, at a depth of 20 cm. The immature stages of insects found in all the organs were taken to the laboratory of General Botany and Plant Physiology of the Politécnico Colombiano Jaime Isaza Cadavid, in plastic boxes with food for their breeding until their adult emergence.
The evaluation of the fruit insects was done by taking 30 ripe fruits at random, obtained from those that fell to the ground after shaking the branches of the tree, discarding the old or mummified fruits. No stratification of the trees were taken into account, because the harvest of the tamarind is traditionally done in the region with the shaking of the tree method. The fruit were taken to the laboratory for reviewing. The insects in juvenile stages, present in the fruits, were grown in plastic boxes until the emergence of adults. Ten individuals of each species were conserved to describe the damage and habits, as well as to observe their possible reproduction and survival. The conditions of the laboratory were of an average temperature of 23.5 °C and a relative humidity average of 52.7%. Four individuals per species were prepared to be identified. The preparation of the Coccoidea and aphids for identification consisted of a clarification with 10% KOH, distilled water, alcohol with different concentrations, xylol, Congo red stain, clove oil and assembled with Canadian balsam (Holman, 1974). The larvae of the moths were bred and identified following the key for Pyraloidea larvae (Solis, 2006). The Insects were deposited in the Entomological Collection of the Entomological Museum Francisco Luis Gallego at La Universidad Nacional de Colombia,with codes MEFLG NC 43608, NC 43609, NC 43610, NC 43611, NC 43612, NC 43613, NC 43614, NC 43615, NC 43616, NC 43617, NC 43618, NC 43619, NC 43620, NC 43621, NC 43622, NC 43623, NC 43624, NC 43625, NC 43626, NC 43627, NC 43628, NC 43629, NC 43630, NC 43631, NC 43632, NC 43633.
Determination of the insect infestation percentage (IP)
In order to determine the IP of the insect species collected from tamarind fruits, were examined using a stereoscope NIKON®. The infestation due to each of the most important insects (C. serratus, S. linearis and the Phycitinae complex) was quantified, and then, the IP of the three together, because some fruit were simultaneously affected by several species.
The IP was calculated following the method of Montes et al. (2012), Montoya-Restrepo (1999), Ripa and Larral (2008) and Suárez et al. (2005), using the equation:
Determination of the grade of insect damage
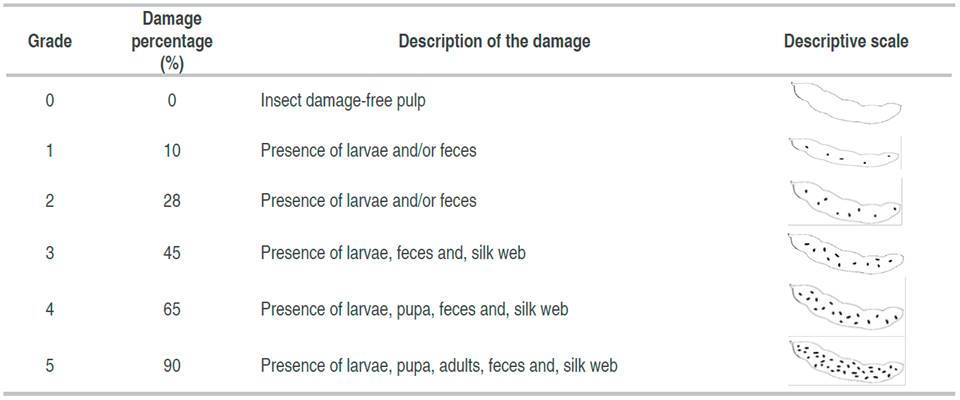
For the analysis of the degree of damage a rating scale of damage was established, obtained from the observation of the harvested fruits, this consisted of ranges of damage from 0 to 5 (0, a healthy organ and 5, an organ with the highest degree of damage) and a graphic scale of these were elaborated according to the affected area in the fruits. Subsequently, a photographic record of each fruit was made, and based on this, the percentage of affected area was determined, using the free access software ImageJ®.
The graphic and descriptive scales of the degree of damage of C. serratus and the Phycitinae complex were made in the pulp (mesocarp and endocarp), which corresponds to the commercial part of the fruit. The scales for S. linearis were made with reference to the seed, since it is there where it does the most damage.
RESULTS AND DISCUSSION
Eleven (11) insects affecting tamarind trees in the region were found. The part of the plant with the highest number of associated phytophagous insects was the fruit, on the contrary, the stem and leaves had the lowest number of species (Table 1). Only the roots were unaffected.
Table 1 Taxonomic identification of phytophagous insects of Tamarindus indica trees in the municipalities of Sopetran and Santa Fe de Antioquia.

Insects causing tamarind fruit damage
Caryedon serratus (Coleoptera: Bruchidae): Are females which deposit eggs on the epicarp of the green pod or inside of it when pods are mature; an egg is 1 mm long, approximately. Once the larva emerges, it penetrates the fruit through the pulp and when it reaches the seed, it starts to consume it causing the main damage to this organ (Figure 2A). Furthermore, larval excretions and waste of the seed consumption contaminate the pulp. The larva may become pupa while it is in the seed, in the pulp or outside the fruit. Pupa is 7.5 mm long, approximately. Finally, the adult emerges and its size is around 7.2 mm.

Figure 2 Phytophagous insects with their respective damage to Tamarindus indica L. fruit; A. Caryedon serratus; B. Phycitinae complex; C. Sitophilus linearis; D. Hypothenemus obscurus.
Morph 1 (Lepidoptera: Pyralidae: Phycitinae): this moth was found depositing eggs on the fruit cover. The eggs are less than 1 mm long. Once the larva emerges, it penetrates the fruit and consumes the pulp while leaving its excretions there, causing the greatest damage to the organ (Figure 2B). Then, the larva becomes pupa on the pulp surface and finally, the adult emerges. Adult moth is 9.2 mm long, approximately.
Sitophilus linearis (Coleoptera: Curculionidae): The life cycle of this weevil occurs inside the seed, where it causes the greatest damage (Figure 2C). The adult has an approximated size of 4.8 mm, it drills the epicarp and the pulp and finally it reaches the seed. And there it starts to consume the endosperm, digging cavities to deposit its eggs. The larva remains at the same site and consumes the seed, where it becomes pupa. The pupa measures 4 mm long. The waste of the consumption in the different stages of the insect cause a little contamination of the pulp.
Ectomyelois ceratoniae (Lepidoptera: Pyralidae: Phycitinae): The larva affects the fruit similarly to morph species 1, however it is bigger than the morph species. E. ceratoniae can be found on the same fruit with morph 1 and it is difficult to differentiate each other with the bare eye.
Hypothenemus obscurus (Coleoptera: Curculionidae: Scolytinae): the adult one is 1.2 mm long. It was found drilling the epicarp (Figure 2D). While passing through the pulp, it leaves its feces and when it reaches the seed, it drills and makes tunnels inside of it.
Percentage in Insect infestation (IP)
The IP of the affected fruit by the three insects, according to the phenotype, was of 28% in the sweet phenotype, and of 21%. In the acid phenotype. C. serratus presented an IP of 17% and of 22%, in acid and sweet tamarind, respectively. S. linearis recorded an IP of 2 and 3%, in acid and sweet tamarind, respectively. While the Phycitinae moths complex showed an IP of 6% for the acid Tamarind and 10% for the sweet tamarind. The average insect IP was 19.5%, 8% and, 2.5% for C. serratus, Phycitinae moths and, S. linearis, respectively (Figure 3).
Damage grade
The descriptive and graphic scales for C. serratus, the Phycitinae complex and, S. linearis are presented on tables 2, 3 and 4, respectively.
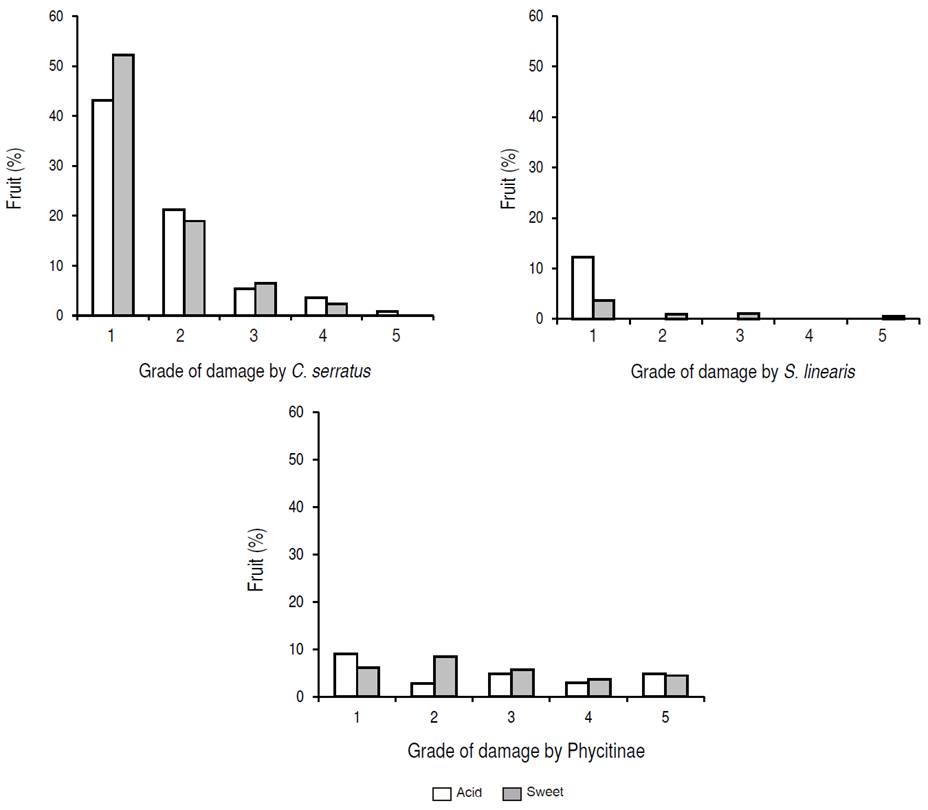
The highest percentage of affected fruit by C. serratus (43% - 52%) was recorded as grade 1 while the lowest percentage of damaged fruit by this pest (0% - 1%) was graded 5. The sweet tamarind showed more affected fruits by C. serratus in different damaged grades (Figure 4).
The highest percentage of tamarind fruit affected by S. linearis (4% - 12%) was registered as grade 1 while the other grades were found in a very low percentage of affected fruits (0% - 1%) by this insect. the acid tamarind fruit was classified in grade 1 (12%), on the other hand, the classification of the sweet tamarind fruit damaged by S. linearis corresponded to grades 1, 2, 3 and, 5.
The highest percentage of fruit affected by the moths of Phycitinae (6% - 9%) had grade 1, and the lowest percentage (3% - 4%) was classified in grade 4. The tamarind acid fruit also presented the highest percentage of insect damage (9%) that had grade 1 and, only 8% of tamarind sweet fruit damaged by this pest was recorded with grade 2. The average percentage of tamarind affected fruits having in the different grades of damage (5.6%) occurred in those from sweet tamarind phenotype.
The number of species found in this study (11) does not overcome those reported in tamarind in Mexico (14) (Orozco, 2001). In the present work, five new record were recorded for T. Indica in Colombia: Ectomyelois ceratoniae, Hypothenemus obscurus, Toxoptera aurantii, Trigona sp. and, Acromyrmex octospinosus. In both Mexico and Colombia, C. serratus, S. linearis and, the Cadra genus (syn. Ephestia) (Orozco et al., 2009; Muñoz and Rueda, 2009) are the most important species affecting tamarind fruit. Actually differing from those registered in other regions of the world (Gupta et al., 2017). This reveals the importance of these coleopteran species as key pest in the neotropical tamarind areas. In addition, in our study, we found two new Lepidoptera species previously unreported in the country.
Caryedon serratus was registered for the first time in tamarind crop in the region where this study was done (Vélez, 1972), in the municipality of Santa Marta (Magdalena department - Colombia). So the continuity of this specie is conformed as an important tamarind insect pest.
Sitophilus linearis was reported in the United States by Cotton (1920), in Mexico by Orozco et al. (2009) and, by Muñoz and Rueda (2009) in the studied region. In this study, it was proved that this insect affects the seed. However, before the seed, the adult passes through the pulp affecting its quality.
The complex of Phycitinae moths reported in this study are different from those moths reported by Muñoz and Rueda (2009) and Muñoz et al. (2014) in the same Colombian region (C. cautella and Amyelois transitella). Thus, it is evident that various Lepidoptera species are pests of tamarind fruit in the nearby western of Antioquia. While the Coleoptera species H. obscurus affects the seed, documented by Wood (2007) affecting the tamarind crop as one of various hosts.
Sweet tamarind may be more susceptible to insect infestation, probably because the trees have very little or none management at all. Moreover, some fruit are not harvested remaining on the trees, stimulating the insect permanence, hence its life cycle and reproduction is not interrupted. This difference in crop management is due to the limited commercialization of sweet tamarind, because the acid one is preferred for making confections that needs sugar.
CONCLUSIONS
In this study, the most important tamarind insect pests with the highest IP were C. serratus (17-22%), the Phycitinae moths (6%-10%) and, S. linearis (2%-3%). In general, the most affected fruits had more than one insect pest, and the degree of affectation was low. Studies on the abundances of the phytophagous insects of the fruit, should be carried out to quantify aspects such as the effect on the phenotype (acid, and sweet tamarind), the management carried out by the producer and the age of the trees, and the insects of the fruit. It is important to evaluate some cultivation practices, and the use of low impact biological insecticides, can contribute to improve the products derived from this fruit. It is also necessary to evaluate the economic thresholds and levels of economic damage, to determine the effect of these insects on the production.