Mango is among the five most consumed tropical fruits worldwide (Caballero et al., 2015). Tommy Atkins is the variety of mango most exported from Ecuador. To be exported, modified atmosphere, hot steam, irradiation, wax coating, and immersion in hot water are used. Mango is normally immersed in hot water to extend its shelf life (National Mango Board, 2020). However, heat treatment accelerates maturation and reduces organoleptic quality.
The short shelf life of mango fruits, susceptibility to chilling injury and postharvest diseases are common postharvest problems that need to be considered for expanding the international mango trade (Singh et al., 2013). Edible coatings become a technological alternative that may reduce mango deterioration during storage. Edible coatings are made from proteins, polysaccharides and lipids. Starch is the most important material used in the formulation of biodegradable films and edible coatings (Chiumarelli et al., 2010). Plasticizers and other additives are utilized to modify the physical and functional properties of edible coatings (Shah et al., 2016).
The use of edible coatings along with essential oils (EOs) has importance because of EOs extend the shelf life of food (Sung et al., 2013). EOs and their active components extracted from aromatic and medicinal plants have antibacterial and antifungal properties (Maurya et al., 2021). Some examples of the EO constituents are carvacrol (found in thyme and oregano), carvone (dill seed), cinnamaldehyde (cinnamon), citral (lemongrass), p-cimene, eugenol (clove), limonene (citrus fruits), menthol (peppermint) and thymol (thyme and oregano) (Kawacha et al., 2021). EOs have been used together with starch coatings to preserve fruits: cassava starch and cinnamaldehyde-thymol in fresh-cut mango (Santacruz, 2021), cassava starch and thyme in apples and persimmons (Sapper et al., 2019), and sweet potato starch and cumin essential oil in pears (Oyom et al., 2022). In addition to EOs, salicylic acid (SA) can be used in the formulation of edible coatings to delay fruit ripening. SA has been used to control fruit decay of guava (Lo'ay and El Khateeb, 2011), apricot (Ezzat et al., 2017) and papaya (Castro et al., 2017). Edible coatings based on chitosan (Chien et al., 2007), arabic gum (Khaliq et al., 2015) and alginate (Robles-Sánchez et al., 2013) have been used in mango, however, no studies on the use of edible coatings based on cassava starch containing SA, cinnamaldehyde or thymol on Tommy Atkins mango have been performed.
In recent years, chitosan has had considerable interest in the industry due to its biodegradability, non-toxicity (Wang et al., 2020a), and antimicrobial properties (Wang et al., 2020b). Studies of chitosan coatings to preserve mango have been performed (Yin et al., 2019; Tavassoli-Kafrani et al., 2020). However, the low availability and high cost of chitosan in the Ecuadorian market (Salas, 2011) compared to other materials may reduce its application. Therefore, essential oils and salicylic acid together with cassava starch could be a good choice for the formulation of edible coatings thanks to its availability and relatively low price (Souza et al., 2012).
In the present work, Tommy Atkins mangoes were coated with either chitosan or cassava starch. Starch coatings together with SA or a mixture of cinnamaldehyde-thymol were utilized. A comparison of total soluble solids, titratable acidity, weight loss, and instrumental texture (firmness) of coated fruits with the two coating materials along four weeks of storage at 12 °C was done.
MATERIALS AND METHODS
Chitosan, degree of deacetylation 95% and Mw of 149 kDa, was donated by Universidad Pública de Navarra, Spain. Cinnamaldehyde, thymol, tween®-20, glycerol, and glucose were obtained from Merck (Germany). Cassava starch (La Pradera, Ecuador) and Tommy Atkins mangoes were obtained from a local market in Manta, Ecuador. Mangoes with a degree of ripening of two (Báez, 1998) were selected according to the size and without any injuries. Afterward, 48 mangoes were washed with water (Santacruz, 2021) and 12 fruits were used for each treatment (Table 1). The coated mangoes were obtained by immersing the fruits in three different solutions (Table 1), followed by drying at room temperature (approx. 25 °C) and storage for four weeks at 12 °C and an RH of 90%. Analyses of weight loss, total soluble solids, titratable acidity, and instrumental texture were performed in triplicate every week.
Coating forming solution preparation
Chitosan coating forming solution was prepared by dissolving 1% (w/v) of chitosan in 1% (v/v) acetic acid solution. 1% (w/v) of Tween 20, 0.5% (w/v) glycerol and 0.5% (w/v) of glucose were added before the solution was homogenized by using an ultraturrax (Polytron, Switzerland) at 11000 rpm for 4 min.
Starch coating forming solution was prepared according to Santacruz et al. (2015). A 0.5% (w/v) cassava starch suspension in water was heated from room temperature up to 90 °C, under stirring, where it was kept for 5 min. 1% of Tween®-20, 0.5% (w/v) glycerol, and 2 mmol L-1 of SA were added before cooling. Once the solution was cooled to room temperature, glucose (0.5%, w/v), 0.15% (w/v) cinnamaldehyde (>95%) and 0.15% (w/v) thymol (98.5%) were added. Finally, the coating forming solution was homogenized by using an ultraturrax (Polytron, Switzerland) at 11000 rpm for 4 min.
Weight loss, titratable acidity, and total soluble solids of coated mangoes along storage time
Weight loss was calculated by the following equation.
Where: WL: Percentage of weight loss, W0: Fruit weight at time zero, Wt: Fruit weight at any storage time.
Titratable acidity was determined by titration with 0.01 M NaOH solution according to the AOAC method (1990), the results of three measurements were reported as a percentage of citric acid. Total soluble solids were determined according to the AOAC method (1990). Three fruits were disintegrated using a household blender and the obtained juice was filtered with textile and analyzed with a refractometer (Atago, Japan). The results of three measurements were reported as °Brix.
Instrumental texture analysis
Puncture tests were performed in triplicate at the central part of three fruits according to Castro et al. (2014). A Shimadzu texturometer (Model EZ-LX, Japan) together with a stainless-steel probe of 3 mm diameter and 8 cm length were utilized. The probe was inserted 15 mm into the fruit at a speed of 10 mm s-1 and the maximum penetration force was recorded.
RESULTS AND DISCUSSION
Weight loss, titratable acidity, and total soluble solids of coated mangoes along storage time.
Mangoes coated with cassava starch containing salicylic acid (SSA) showed the highest weight loss, whereas fruits coated with starch-cinnamaldehyde-thymol (SCT) showed the lowest weight loss throughout the whole storage time (Table 2). SCT mangoes showed a weight loss that varied between 4.4 and 15.7%, which was statistically different than SSA samples. After the first week of storage, no difference in weight loss was found among uncoated mangoes and mangoes coated with either chitosan or SCT. The hydrophilic nature of starch may contribute to losing water in a higher amount in mangoes coated with SSA compared to uncoated samples. The use of cinnamaldehyde-thymol together with starch (SCT) led to lower weight loss compared to SSA. The hydrophobic properties of cinnamaldehyde-thymol (Man et al., 2019) may be the reason for such behavior. The incorporation of EOs into the coating-forming solution can confer water-resistance properties to coatings because this oil has a hydrophobic nature (Sánchez-González et al., 2011).
Table 2 Weight loss and total soluble solids of coated mangoes. Coatings based on either chitosan or cassava starch and stored for 28 days at 12 °C and 90% relative humidity.

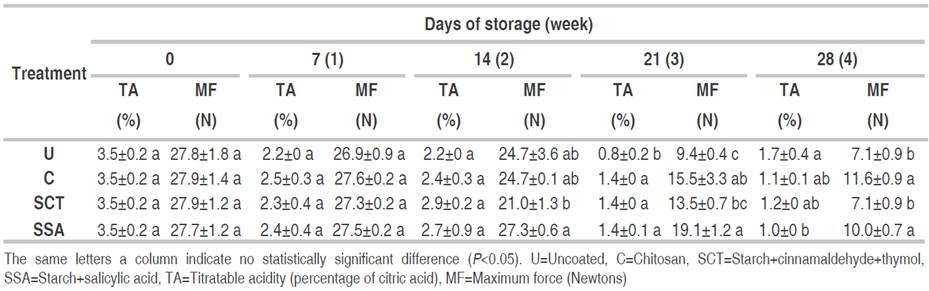
Titratable acidity showed a decrease along the four weeks of storage. Biochemical changes, e.g., ascorbic acid content on mangoes during ripening may lead to a reduction of titratable acidity (Pandarinathan and Sivakumar, 2010). There was no difference in titratable acidity among samples up to the second week of storage (Table 3). However, on the third week of storage, mangoes coated with chitosan, SSA, and SCT samples, ripened more slowly as indicated by higher acidity than uncoated samples. On the fourth week of storage, only mangoes coated with SSA showed lower acidity than the uncoated sample. A decline in acidity demonstrates advancement of maturation (Maftoonazad et al., 2008), thus the coated fruits contributed to delaying the fruit maturation/ripening. Higher acidity in coated fruits may be the result of the formation of carboxylic acid by dark fixation of CO2 (Maftoonazad et al., 2008).
Table 3 Titratable acidity and maximum force of coated mangoes. Coatings based on either chitosan or cassava starch and stored for 28 days at 12 °C and 90% relative humidity.
The four treatments showed an increase in total soluble solids along the storage time. Total soluble solids had no differences among samples up to the first week (Table 2), however, an increase for the four samples during the second week of storage was noticed. At the end of storage, the uncoated sample reached the highest value of total soluble solids (approximately 14 °Brix), which was statistically different than the three coated mangoes (total soluble solids between 9 and 11 °Brix). No statistical difference was found among total soluble solids for coated mangoes. The low value of mangoes coated with SSA suggests that SA may reduce the rate of ripening. This ripening reduction may be probably through inhibition of ethylene biosynthesis (Yin et al., 2013). In fact, Lo'ay (2017) found that an exogenous supply of SA delays the ripening of grapes.
Texture analysis
Results of puncture tests showed that the maximum force of penetration decreased during the storage time for all samples. Mangoes coated with SSA and chitosan showed statistically similar forces of penetration which were also higher than SCT and uncoated samples (Table 3). SSA and chitosan coatings led to mangoes with low total soluble solids and low penetration forces which may be the result of a low respiration rate (Cissé et al., 2015) and an inhibition of ethylene production by the presence of SA (Hayat et al., 2007). Chitosan coating caused substantial delays to some processes involved in ripening most notably weight loss, total soluble solids, and texture of the fruit. These effects may be linked to the reduced rates of ethylene production and respiration that might be the result of lower internal oxygen levels (Jitareerat et al., 2007).
CONCLUSIONS
The use of coatings based on either chitosan or cassava starch containing either salicylic acid or cinnamaldehyde-thymol delays changes in weight loss, soluble solids, titratable acidity, and firmness of mango along four weeks of storage at 12 °C. Cinnamaldehyde-thymol improves the hydrophobic characteristics of the cassava starch coating compared to salicylic acid and therefore reduces the weight loss of stored mango. For practical applications, the use of starch coatings with salicylic acid together with cinnamaldehyde-thymol to control the ripening of mango could be analyzed in future research.

















