The pitahaya is a cactus, native to the Andean region of the tropics and subtropics of Central and South America, mainly cultivated in Bolivia, Ecuador, Colombia, and Brazil (Vilaplana et al. 2018). Pitahaya cultivation in Colombia has a high commercial value as an export crop because it is an exotic fruit known as dragon fruit in markets such as Japan, Europe, the United States, and Canada (Agronet 2022). The yellow pitahaya is a fruit with a high demand for its unique appearance, flavor, quality, and nutritional properties in vitamins, antioxidants, high fiber, flavonoids, and phenol contents (Ibrahim et al. 2018; Betancur et al. 2020; Pásko et al. 2021; Da Graca et al. 2023).
Colombia is the ninth supplier of this exotic fruit worldwide. The country has 827 planted areas, it produced 124.5 million tons and 17 thousand tons which represented 38% of the total international exports (Agronet 2022). In 2009, Colombia was the main producer of pitahaya in the world (FAO 2009). The Boyacá's department is the main producer with about 440 hectares planted. Hence, in the municipality of Miraflores, in Boyacá there are 53 farms producing pitahaya, with a cultivated area of 42 hectares (Morillo-Coronado et al. 2022).
The most widely planted genotypes currently in production systems of Boyacá, Colombia is the yellow pitahaya, Hylocereus megalanthus known as Selenicereus megalanthus (Morillo-Coronado et al. 2021). One of the limiting factors in the production of the pitahaya crop is the diseases caused by phytopathogens and can cause losses in productivity because the bacteria can infect plants during processes of establishment and growth of the crop. The phytopathogens can infect stems, developing fruits, and post-harvest fruits (Balendres and Bengoa 2019; Lozada et al. 2022). However, there are few official reports on the composition of the bacterial community in pitahaya diseases (Peng et al. 2022). The pitahaya is vulnerable to bacteria, fungi, viruses, and some insect pests and it has been reported 17 genera and 25 species of phytopathogens. The bacterial phytopathogens identified are Enterobacter cloacae, Enterobacter hormaechei and Paenibacillus polymixa (Balendres and Bengoa 2019). Enterobacter cloacae has been reported to cause disease in Malaysian crops (Masyahit et al. 2009), while Paenibacillus polymixa was identified as the cause of soft rot in China (Zhang et al. 2017). Enterobacter cloacae has been detected in H. undatus in Peru (Soto et al. 2019). The first report of stem rot in H.costaricensis for Costa Rica identified the bacterial isolation of Enterobacter hormaechei (Retana et al. 2019).
Although the main producer of pitahaya in Colombia is the department of Boyacá, there is a lack of technical and scientific research studies that can improve the agronomic practices of farmers in the sector (Gaona et al. 2015). Likewise, there are no official reports of the disease-causing agents for pitahaya crops in the country. Agronomic practices are based on the empirical knowledge of the farmer and the conversion to techniques of integrated disease management can be a solution to the problems (Gaona et al. 2015). In Colombia, phytosanitary problems have been described such as basal rot of the stem and fruit caused by Fusarium oxysporum, dry rot of the stalk caused by Dreschlera cactivora, Anthracnose caused by the fungus Colletotrichum, bacteriosis presumably associated with Erwinia, and the nematodes Meloidogyne (Burgos 2013; Gaona et al. 2015; Salazar-González et al. 2016). There are no official reports of the identification of bacteria isolated from signs and symptoms of soft rot for the region. Due to the lack of official publications, this research aimed to analyze the etiology of the disease, identify and characterize the composition of the cultivable bacterial community associated with soft in yellow dragon fruit (Selenicereus megalanthus haw.) of Boyacá, Colombia.
MATERIALS AND METHODS
Sampling locations
The study was done in 13 yellow pitahaya farms with open field production systems and covered crops (S. megalanthus) located in the Municipally Miraflores, Department of Boyacá (Colombia) (05°14'121" N; 073°11'949" W). The region has an average annual temperature of 19.5 °C and a relative humidity of 88.9%.
The soil fertilization management is rich in major elements. The farmers do a chemical control of diseases with Oxychloride (80 g 20 L-1), the curative and preventive contact iodine liquid Baladine® (100 mL 20 L-1) or powder (80 g 20 L-1). Diseases with high incidence included basal rot, fungal symptoms and signs with unidentified microbial agents, and pests included the flower bud fly (Dasiops saltans) and the potato bug (Leptoglossus zonatus).
Sampling
Pitahaya plants in the initial stage of flowering and full fruit production were sampled. The sampling was random, selecting material according to the observation of signs and symptoms in the tissues. A minimum of 20 samples were collected per farm. Samples of stems and fruits with chlorosis, yellow to brown halos, and aqueous tissues due to rotting, were collected mainly in the initial stages of the disease. The plant material was placed in plastic bags and placed in portable refrigerators (0 to 4 °C) and taken to the laboratory. Photographs were made during the sample collection process, identifying the signs and symptoms of bacterial disease.
Isolation of bacteria associated with symptoms
Bacterial isolations using cultivable microbiological techniques were obtained from the symptomatic cladodes and fruits showing lesions. The surface of the tissues was disinfected with 70% alcohol for 1 min and rinsed with sterile water. Pieces approximately 1 mm wide and no more than 3 mm long were cut from the lesion and mixed with a few drops of sterile distilled water for 2 to 4 min to allow bacteria to flow into the fluid. Likewise, the tissues were placed in a sterile 0.85% saline solution and vortexed for 2 min. The aliquotes were taken from this suspension with a bacteriological loop and streak plates of nutrient agar (Merck®) [Composition: Peptone (5 g L-1), meat extract (3 g L-1), NaCl (8 g L-1), bacteriological agar (15 g L-1)].
A 100 µL aliquot was also taken and streaked with a Driglasky loop. The plates were incubated at 30 °C for 24 to 48 h. Controls were set up to discriminate epiphytic microbiota. Sterile swabs were passed over the surface of the tissue and immersed in 0.85% saline solution. 100 µL aliquots were plated on nutrient agar and incubated at 37 °C during 48 h. Epiphytic colonies were discriminated from the isolates obtained in diseased tissues. Five samples of diseased tissue per farm were analyzed. Five pieces were taken from each sample of diseased tissue and planted in triplicate. In total, 65 samples were analyzed and around 200 culture media were planted. The different colonies were plated again on nutrient agar plates and this process was repeated until purified bacterial cultures with homogeneous colony morphology were obtained.
Biochemical and molecular identification of bacterial isolates
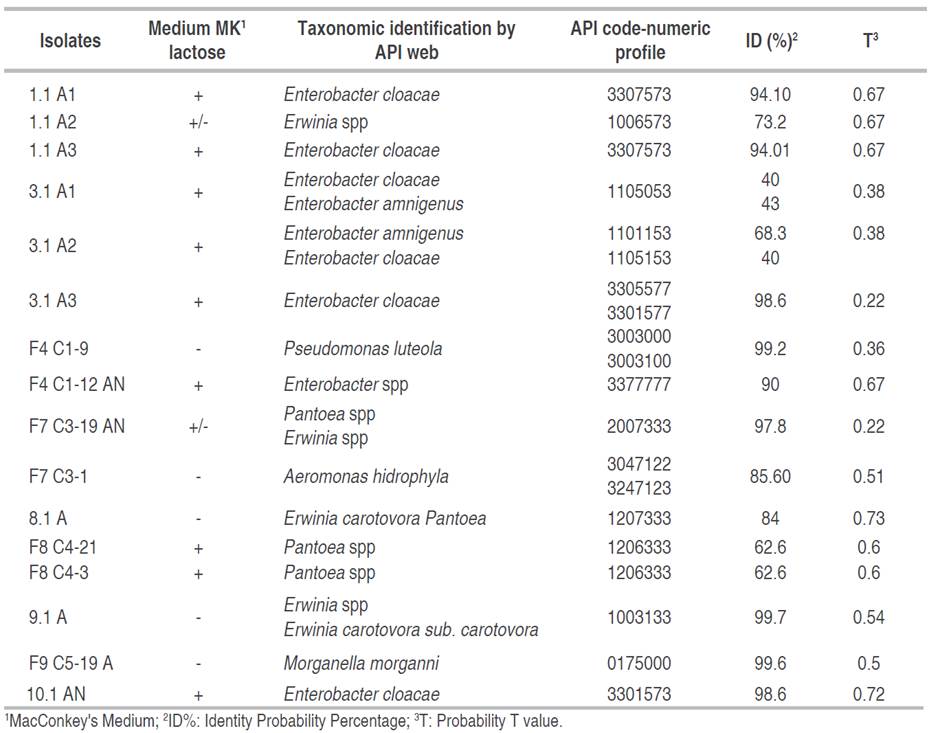
Microscopic morphology was performed using Gram staining and Gram-negative colonies were cultured in MacConckey medium (Merck®). Biochemical identification was performed using the API 20NE® and API20 E® technique (BioMérieux, France) according to the manufacturer's instructions. The following biochemical tests were made: fermentation or oxidation of carbohydrates such as glucose, mannitol, sorbitol, sucrose, arabinose, and rhamnose. It also includes gelatin, tryptophan to produce indole-acetic acid, cytochrome oxidase, arginines, urease test, and the production of acetoin (Voges-Proskauer), among others. The results obtained from the negative or positive reactions were transformed into a 7-digit code called numerical profile entered into the Apiweb® [CD-ROM] BioMérieux software (2010). For the identifications, the Percent Probability of Identity and the T probability value were evaluated. The bacterial strains were preserved in 20% glycerol at -20 °C and in cryovial with conservation beads.
For molecular characterization and identification, the selected bacterial strains were subjected to analyses of 16S ribosomal RNA gene by V2-V5 region sequencing. The selected bacterial strains were grown in trypticase soy agar (Merck®), and the DNA was extracted using DNeasy UltraClean Microbial Kit MoBio®. The 16S rRNA genes were amplified by PCR using the 337F (5' GACTCCTACGGGAGGCWGCAG 3'), 518F (5' CCAGCAGCCGCGGTAATACG 3'), 800R (5' TACCAGGGTATCTAATCC 3'), and 1100R (5' GGGTTGCGCTCGTTG 5'). The amplification of the V2-V5 region of the 16S rRNA gene was done in a final volume of 20 μL, using 100 ng of genomic DNA as a template, and with a concentration of: 0.5 mM of each of the oligonucleotides, 200 μM of each of the 4 dNTPs (dATP, dGTP, dCTP, and dTTP), 1X GoTaq Flexi Buffer, 2.5 mM MgCl2, and 1 U of GoTaq® Taq Polymerase. The PCR cycle was made in an ESCO® SWT-MXB-1 thermocycler under the following conditions: i) One denaturation cycle of 1 min at 94 °C, ii) 30 amplification cycles: 30 s at 94 °C, 30 s at 55 °C and 1 min at 72 °C and iii) final extension of 10 min at 72 °C. The purification process of the PCR fragments and sequencing was done using the Sanger method. The 16s rRNA gene sequences of the V2-V5 region were edited by removing the primers, assembling and obtaining the consensus sequence. The DNA sequences obtained were analyzed with the basic sequence alignment (BLAST) run against the database from the National Center for Biotechnology Information Blast (www.ncbi.nlm.nih.gov/BLAST). The taxonomic analysis of the sequence was made using the "Classifier" and "SeqMatch" tools, hosted on the RDP (Ribosomal Data Project) website (https://bio.tools/rdp). Next, the multiple alignment was made using the Multiple Sequence Comparison by Log-Expectation (MUSCLE) algorithm with the thirty most similar bacterial sequences reported by Basic Local Alignment Search Tool (BLAST) in NCBI, for the generation of a phylogenetic tree using the model of Tamura-Nei genetic distance, with the "Neighbor-Joining" method and the "Bootstrap" method with a thousand replicates. The phylogenetic analysis was done by multiple alignment using the MUSCLE algorithm and the Tamura Nei distance method in the statistical program Geneious prime (2024.0.5).
In-vitro pathogenicity assay and bacteria in insect vector
A pathogenicity assay was done to evaluate Koch's postulates to determine the causal agents of the observed symptoms. The cultures of the Pectobacterium and Enterobacter cutured in nutritive agar medium (Merck®) were suspended in sterilized water (108 CFU mL-1). Four pitahaya stems 4 to 6 cm in diameter were inoculated with 20 μL bacterial suspension on each stem by injection at two opposite locations with a depth of 2 mm. For the control, stems were injected with sterilized water. The inoculated stems were wrapped in clear plastic and incubated at 26 °C for 5 days before the observation of rotting symptoms. Since no pathogenic response was obtained with the first infection procedure, a second infection test was performed. Healthy pitahaya stems were cut, washed with sterile distilled water, disinfected with 1% sodium hypochlorite and finally rinsed with sterile distilled water. The stems were placed in Petri dishes and pierced in several places with toothpicks impregnated with the bacteria. Control stems were pierced with the tips of toothpicks containing Nutrient Agar only. They were sealed and incubated at 30 °C for 72 h.
To verify a possible relationship between insects as vectors of phytopathogenic bacteria, a direct collection of 10 insects of the species Leptoglossus zonatus (Dallas) (Hemiptera: Coreidae) was made up. This insect causes damage to the flower buds and cladode in yellow pitahaya (Medina and Kondo 2012). Each collected insect was deposited alive in sterile glass jars. In a laminar flow chamber, live insects were picked up with sterile forceps from the midsection (between the thorax and the abdomen) and their legs were brought into contact with the Trypticase soy agar culture medium. The plates were incubated at 28 °C and examined daily for bacterial growth that occurred at 48 h.
RESULTS AND DISCUSSION
Description of signs and symptoms of the bacterial disease
The affected plants with brown and yellow spots were observed more frequently. Some cladodes is shows liquefaction, with strong bacterial odors, and postharvest fruits with yellow-brown lesions (Figure 1). The symptoms can begin with small chlorosis of the stems, and it can spread throughout the entire area of the cactus pads, to later generate softening of the stem, dark brown coloration with odors of bacterial rot.
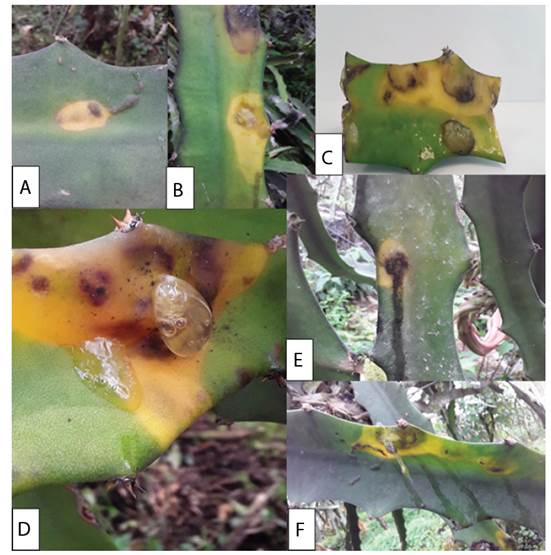
Figure 1 Initial stages of symptoms associated to bacterial disease in Pitahaya (Selenicereus megalanthus Haw.). A-B) First symptoms of the disease, with forms of yellow chlorotic halos can start in a black spot. C) Blister formed from the disease. D) The clearest sign of the disease is the discharge of a mucilaginous fluid developed in the symptoms of chlorosis and blisters. E-F) Mucilage can run down the stem and flows from the lesions.
From the observations and photographs, the possible development initial stages of the disease were reconstructed (Figure 1). The bacteria enter the peduncles through a vector during the first stages related to the biotrophic phase of the microorganism. The microorganisms could enter by small drops of water or by exposure to the mucilage of another diseased plant, and forming symptoms like yellowish chlorotic halos on the stems (Figure 1A). Initial yellow spots have been described as the initial symptoms when injecting Enterobacter pathogens into Pitahaya, which can take on yellow and orange colors after eight days of infection (Retana et al. 2019). The yellowish colorations or chlorotic symptoms spread along the surface of the stem with black borders on the outside of the diseased tissue (Figure 1B, C). Valencia et al. (2003) reported an unidentified Enterobacteriaceae as the causal agent that in the initial stages produces a yellow chlorotic halo. The starting point of chlorotic spots can be in the center of the stem or edges. For example, Soto et al. (2019) describe that initial yellowish or chlorotic spots may begin on the protruding edges of the stem and may extend to the center of the stem.
Subsequently, the injured tissue becomes inflamed, forming a blister that accumulates a translucent liquid, with a mucilaginous appearance or mucus. The fluid is secreted and can run down the entire surface of the stem. This fluid may be a mechanism of persistence and dispersion of the bacteria (Figure 1D, F). Meanwhile, Masyahit et al. (2009) describe several isolates of the Enterobacteriacea family such as Enterobacter, Pantoea, Klebsiella in symptoms of yellow chlorosis that the symptoms caused by Enterobacter do not affect vascular bundles, but yellow and dark brown chlorotic halos can develop.
In the final phases of the development of the disease, the tissue can show liquefaction, strong odors related to bacterial growth, and extensive decomposition in the tissue. It would be the final necrotrophic phase of the bacteria. In the final symptoms of the disease, some cladiodes stop the progression of the disease, while in others the necrosis is total (Soto et al. 2019). The arrest of symptoms in a stem can be caused by the high calcium contents that the plant can store in the form of oxalate (Faheed et al. 2013; Retana et al. 2019). Furthermore, Soto et al. (2019) describes a decomposed stems with strong bacterial odors detach from healthy tissue under their own weight, and the plants can keep vascular bundles and epidermis intact attached to healthy stems.
Bacteria identified in soft rot
A total of 25 bacterial isolates were cultured. Microscopic morphology was performed by Gram stain, described 16 strains corresponding to Gram-negative bacilli, and these were cultured twice in MacConkey agar and conducted biochemical tests using API 20E® and API 20NE® kit (BioMérieux, France) (Table 1). Genera such as Enterobacter, Erwinia, Pseudomonas, and Pantoea were identified with a high percentage of identity from the biochemical tests According to the API code (Table 1). The Enterobacter strains can produce β-galactosidase, arginine dehydrolase, ornithine decarboxylase and gelatinase in this metabolism. Likewise, the Enterobacter strain produce acetoin and can oxidize or ferment citrates, glucose, mannitol, inositol, rhamnose, cellobiose and arabinose. This metabolic versatility could be used by the phytopathogen in the biotrophic phases to penetrate cell walls by liquefaction of pectins and in the necrotrophic phase to take advantage of the photosynthate organic compounds of pitahaya. Aerobic chemoheterotrophy and oxydative functional group have been identified in the core microbiome of soft rot by metagenomic function prediction, suggesting that plant pathogenic bacteria need to break down tissue organic matter in the early stages of plant-pathogen interaction (Peng et al. 2022).
The cultivable microbiota of the pitahaya's soft rot in Boyacá, Colombia is composed by 25 morphotypes grouped into 4 phyla (Firmicutes, Actinobacteriota, Bacteroidota, Proteobacteria or Pseudomonadota), 6 classes, 8 orders, 9 families, 13 genera and 25 morphotypes (Figure 2). Bacteria identified by ARNr 16S gene sequencing were clustered with 97-99% relationship with other type strain or ATCC reference sequences from the gene bank based on the phylogenetic and taxonomic analysis by BLAST in NCBI and the Ribosomal Data Project (Figure 2). Four strains studied corresponded to the genus Enterobacter (two E. cloacae, and species like E. ludwigii). Enterobacter strains were isolated from stem lesions and postharvest fruit. The two strains Pectobacterium carotovora were isolated from diseased stems. Likewise, Paenibacillus glucanolitycus is identified in the symptoms of chlorosis in stems and soft rot of fruits.
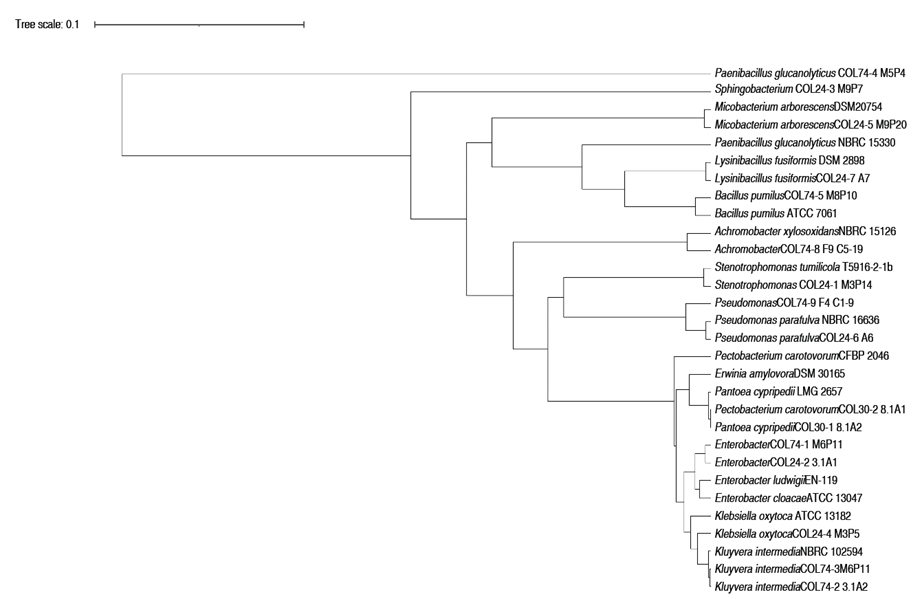
Figure 2 Phylogenetic tree based on 16S rRNA gene sequences of bacteria isolated in signs and symptoms of soft rot from Selenicereus megalanthus crops from Boyacá, Colombia. The COL initials of the strain identification code refer to the bacteria isolates for Colombia. The Variable regions of the 16S rRNA gene were sequenced with primers 1100R-337F and 800R-518F. The tree was constructed using the genetic distance model of Tamura Nei and UPGMA Tree building method.
This study demonstrated the association of three bacterial species with the disease symptoms reported in the literature with soft rot of the Pitahaya stem: Enterobacter cloacae, Pectobacterium carotovora and Paenibacillus glucanolyticus. In addition, two new species are reported as possible phytopathogenic bacteria of pitahaya: Pantoea cypripedii and Enterobacter ludwigii. It is important to highlight how four genera of bacteria (Enterobacter, Pantoea, Kluyvera, Erwinia and Pectobacterium) belonging to the Enterobacteriales order were identified in the symptoms of the bacterial disease in Pitahaya. The three closely related families (Enterobacteriaceae, Erwiniaceae and Pectobacteriaceae) are part of Enterobacteriales, assigned from the very close phylogenetic clades of Enterobacter-Escherichia, Erwinia-Pantoea, Pectobacterium-Dickeya (Adeolu et al. 2016). This result may suggest an enterobacterial complex causes diseases Pitahaya crops and a possible coevolutionary relationship in the plant-pathogen interaction. Enterobacteriaceae soft rot (ESR) has been identified by different genera according to the taxonomic assignments made for some strains, from Erwinia (in 1917) to some strains classified as Pectobacterium and Dyckeya (in 1945) and currently as Pantoea (Brady et al. 2010; Charkowski et al. 2014; Adeolu et al. 2016). Different bacterial isolates classified in the three genera have been reported as causative agents of soft rot in tubers, rice, corn and brassicas (Charkowski et al. 2014).
The Enterobacter strains have been frequently reported as a phytopathogen of pitahaya. Masyahit et al. (2009) isolated Enterobacter cloacae from pitahaya stem infected with soft rot. Isolates bacterial of Enterobacter cloacae, Enterobacter nimipressuralis, and Enterobacter pyrinus have been identified in soft rot of Hylocereus undatus from China (Lin et al. 2015). In Costa Rica for Hylocereus costaricensis and Hylocereus undatus in Peru, Enterobacter hormaechei and Enterobacter cloacae was isolated as the causal agent soft rot, respectively (Retana et al. 2019; Soto et al. 2019). In the study by Peng et al. (2022), samples of soft rot symptoms in Hylocereus were analyzed using metagenomic libraries of the 16s rRNA gene. These researchers have detected a high abundance of operational taxonomic units (OTU) of Enterobacter and Pseudomonas as predominant genera in the development of the disease. Similarly, Enterobacter strains have been identified as endophytic bacteria in pitahaya seedlings that can induce typical soft rot symptoms (Lin et al. 2015). It is interesting how Enterobacter species have been reported as plant growth-promoting bacteria (Ogbo and Okonkwo 2012), as commensal and beneficial endophytic bacteria (Grimont and Grimont 2006). However, they have also been reported as phytopathogenic bacteria (Soto et al. 2019). The change of a bacteria between two behavioral states (asymptomatic endophyte and pathogenic) is interesting because a microbial group can transit in the middle of a commensalistic relationship with the plant (Stengel et al. 2022). This theory can be explained by the concept of continuum endophyte. Certain bacterial taxonomic groups can exhibit pathogenic or mutualistic traits depending on the environmental niche, the phenological state of the plant, and the association with the host (Stengel et al. 2022). This theory can be explored in the future research in the ecological system of pitahaya-Enterobacter.
The genera Achromobacter, Pseudomonas, Klebsiella, Stenotrophomonas, Microbacterium, and Sphingobacterium were identified by molecular techniques like the microbiota associated with stem symptoms, possibly as endophytes, phytopathogenic, necrotrophic or environmental bacteria (Figure 2). Pierangeli (2019) also identified three endophytic bacteria that reside on host plants without causing symptoms like Microbacterium (M. arborescens, M. testaceum and M. imperial), Pseudomonas rhodesiae, Enterobacter asburiae, and Bacillus altitudinis by the Maldi-TOF MS technique in fresh pitahaya pulp (Hylocereus undatus) with lesions of gelatinous spots. This genera are considered biological control agent and/or plant growth promoting bacteria. These microorganisms are beneficial to the plant and can establish mutualistic interactions like endophytic bacteria; however, during the development of a disease, in tissue decomposition, they can take advantage of nutritional resources such as carbon and energy sources to proliferate in lesions. Further study of the interaction of these strains in the development of the disease is necessary, since genera such as Pseudomonas, Paenibacillus, Microbacterium and Sphingobacterium have been reported as the most abundant in the bacterial community of the disease during the late stages or necrotrophic phase (Peng et al. 2022). This suggests a rol like pathogens or opportunistic copiotrophic bacteria. Equally, in pitahaya stem tissues affected by soft rot caused by Enterobacter, non-pathogenic bacteria have also been identified such as Klebsiella mobilis, K. oxytoca and Pantoea dispersa (Masyahit et al. 2009). In this research, Achromobacter, Pseudomonas, Klebsiella, Stenotrophomonas, and Microbacterium were identified as endophytic microbiota accompanying the development of the disease, and they could be heterotrophic, opportunistic, and copiotrophic bacteria that appear in the decomposition process in the necrotrophic phase of the soft rot. In addition to the isolated bacteria, fungi of the special Fusarium fujikoroi Complex (in the publication process) were isolated from some of the liquefied lesions and it is a pending report to be made.
It is interesting how some genera identified in this study could play an important role in infectivity. For example, Sphingomonas, Enterobacter and Pseudomonas could play an important role in the biotrophic stage of the disease. Their increase is related to chemo-heterotrophy, heterotrophy and symbiotic or parasitic interaction with the plant. During the development of the disease there is a change in the composition of the disease. Enterobacter is the genus that could be guiding or modulating the response of the pathogenic functional groups (Peng et al. 2022). The role of Pseudomonas is still not clear, since in healthy pitahaya it has been reported as abundant together with the Enterococcus genus (Peng et al. 2022). It would be interesting in future works to evaluate strains of these genera as biological control agent of soft rot.
Analysis of phylogenetic relationships of the bacterial genera identified in stem rot
The results of the taxonomic analysis for Paenibacillus's DNA sequence against the NCBI ref_seq database, indicate that it has 99% identity in 97% of its length, with 16S ribosomal gene sequences, belonging to the species P. glucanolyticus and P. lautus. The distance tree (unpublished results) constructed from the thirty closest culturable microorganism sequences available in the NCBI RefSeq_RNA database shows clusters with sequences from the species Paenibacillus glucanolyticus. There is previous evidence of the participation of Paenibacillus polymyxa in lesions of the pitahaya species Hylocereus undulatus, eventually turn yellow and brown color, where finally the fleshy stems completely decompose, leaving only the woody pith stem center (Zhang et al. 2017).
This study could be considered the first scientific proof of Pectobacterium carotovora in soft stalk rot in yellow pitahaya in Colombia. Pectobacterium is known to cause destructive soft rot disease in many economically important vegetables such as carrots, cabbage, cucumbers, onions, pepper, potatoes, and tomato (Adeolu et al. 2016). Likewise, the Pectobacterium genus could be causing secondary infections because it is a necrotrophic bacterium actively kills host tissue as it colonizes and thrives on the contents of dead or dying cells (Davidsson et al. 2013).
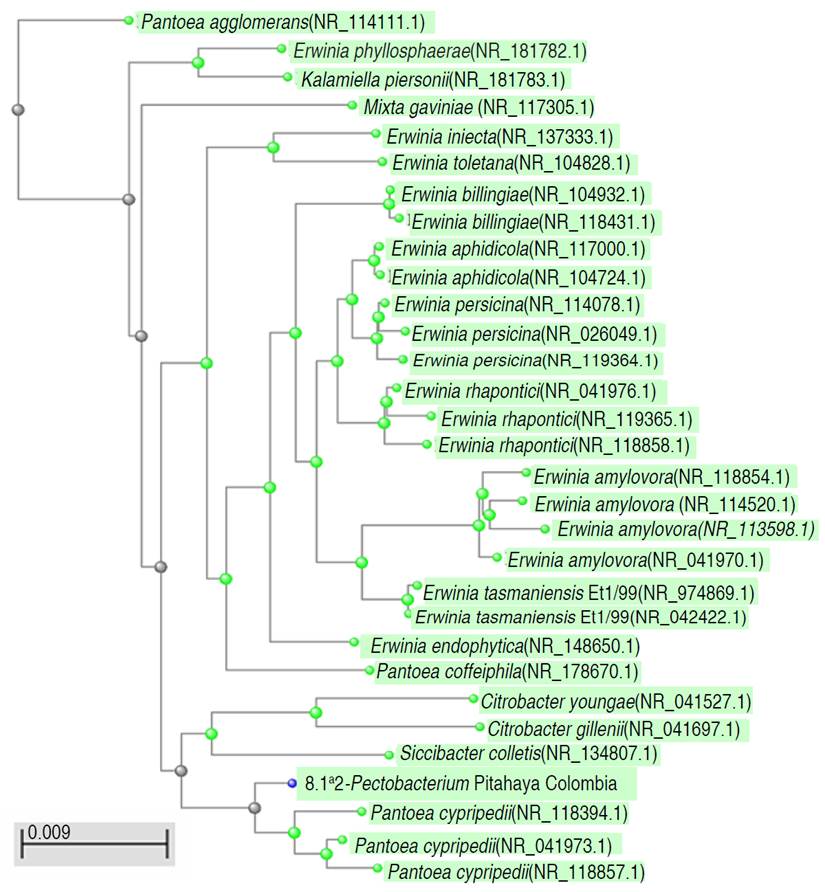
A bacterial isolate identified as Pectobacterium spp. by biochemical test and RDP was classified within Erwiniaceae family (Figure 3), with a high identity percentage, possibly classified as an Erwinia amylovora species. The assembled test sequence presents greater homology with sequences from the genus Erwinia spp. However, the results of the taxonomic analysis of the 1,487 bp assembled query sequence against the NCBI ref_seq database indicate that it has a 99% identity belonging to the species Pantoea cypripedii, previously taxonomically classified as Pectobacterium cypripedi. It is considered the first report of the possible phytopathogenic strain of Pantoea cypripedii for Colombia identified in Pitahaya. It is necessary to deepen the investigation of this species and to review their taxonomic assignments. Brady et al. (2010) described the Pantoea genus became part of the Erwinia herbicola-Enterobacter agglomerans complex, with more phylogenetically close Pectobacterium cypripedii strains such as Pantoea. However, the Pectobacterium cypripedii species became part of Pantoea, as Pantoea cypripedii. This is an example of how the molecular taxonomic identification of phytopathogenic strains can change depending on the genomic databases used and the taxonomic reassignments of the genera studied. For a more robust taxonomic and phylogenetic analysis and to distinguish between species of Pantoea, sequence analysis of other genes (rpoB gen, gyrB gen, atpD gen) is necessary in an Assessment of Multilocus Sequence Analysis (MLSA). The phylogenetic relationships of the order Enterobacteriales are still not clear, within the order Enterobacteriales there are three close families: Enterobacteriaceae, Erwiniaceae and Pectobacteriaceae (Adeolu et al. 2016). The taxonomic reassignments of the strains belonging to the genera Pectobacterium as Pantoea, and the classification of some strains of Erwinia like Pantoea have been described, and it is related with the formation of different clades between Erwinia-Pantoea and the Pectobacterium-Dickeya clade (Adeolu et al. 2016). Described and discussed above, the strain identified in this study corresponds to Pantoea cypripedii. The genus Pantoea has not been detected as a pathogenic bacterium for pitahaya Colombian crops, and it may be part of the endophyte bacterium and accompanying microbiota of bacterial symptoms (Masyahit et al. 2009). However, Pantoea species are pathogenic of corn, onion, rice, and eucalyptus and can proliferate in various niches and cause diseases in a wide range of hosts (Weller-Stuart et al. 2017). The above discussion can be observed in Figure 2. The Pantoea and Pectobacterium isolates recorded in this research in Colombia have a phylogenetic closeness as they are grouped in the same clades together with reference strains of the same genus and Erwinia.
Pathogenicity tests
None of the plants inoculated with Enterobacter cloacae and Pectobacterium carotovora and bacteria or with sterile water had any malformation symptoms. Although Koch's postulates could not be effective to relate as a causal agent of the symptoms of bacterial disease, some of the bacterial genera identified in this study have been reported as causal agents of the disease in different dragon fruit crops. Likewise, in most inoculation assays of phytopathogenic bacteria in pitahaya, it has not been possible to achieve adequate development of symptoms and infectivity because the environmental factors involved in the phytopathogen system are still unknown (Salazar-González et al. 2016; Peng et al. 2022). Likewise, the infectivity protocols have been used in assays with dragon fruit of the genus Hylocereus and have not been described for Selenicereus infectivity assays. It is necessary to design new infectivity protocols, changing variables such as the density of the bacterial inoculum, temperature, humidity, and inoculation mechanisms. In the literature there is little evidence of positive results in a different species of pitahaya. Thus, the in vitro pathogenicity test in Hylocereus spp. was positive in caused soft rot symptoms for Enterobacter cloacae (Masyahit et al. 2009). The symptoms appeared 24 and 48 h after inoculation in fruit and stem. The pathogenicity test was 100% incidence in stems infected by E. cloacae and E. hormaechei for H. undatus and H. costaricensis, respectively (Masyahit et al. 2009; Retana et al. 2019; Soto et al. 2019).
For future research, it would be important to evaluate inoculations with consortia of phytopathogens. Possibly in the development of the disease is caused by communities of microorganisms that infect together. Also, is important to improve the conditions of infectivity with plant growth rooms in greenhouses with environmental conditions of humidity, temperature and simulated rainfall, and test other inoculation techniques like the wounds caused by possible vectors.
Agronomic practices and vectors of bacterial diseases
All bacterial isolates were obtained from production systems under a plastic cover, in a greenhouse-type design, regardless of whether the plants were planted in stone, cement, or wood systems. An interesting fact was not identifying signs, symptoms, or achieving isolates in those farms with good cultural management of diseases such as removal of plant material with symptoms, burning and burial with dolomite lime of diseased plant material. A good practice identified was the use of liquid biofertilizers called "mountain microorganisms", mainly based on Trichoderma and Beauveria's fungic inoculant. In crops with this biological control practice there was a low incidence of the disease. Cultural management and biological control of a disease are important as strategies within the intergrade management of the crop that can reduce the incidence and severity of bacterial disease in a crop (Balendres and Bengoa 2019).
The predominance of the disease in some crops can be caused by mechanical wounds to the stems from agricultural tools, insect bites, or the environment (Masyahit et al. 2009). These lesions are an entry point for bacteria that infect the plants. In environments with greater atmospheric humidity and nutrient-rich soils or with crops with foliar fertilizations, these are ideal conditions to increase bacterial growth. Mainly, most of the plant material studied presented open lesions generated by the pruning of the stems. The identified bacteria may be in the crop environment such as water and soil and could enter open lesions through contaminated work materials. Likewise, bacteria can be transmitted by vectors such as flying insects.
Possible vectors of bacterial disease in pitahaya (Selenicereus megalanthus Haw.) are described in Figure 4. The Diptera are attracted to and feed on mucus or mucilage (Figure 4A). Likewise, the ants walk through the chlorotic stains and secretions and feed on the mucilage (Figure 4B). An unidentified larval stage inside the stems during the initial stages of the disease was observed. Small galleries and paths of the larva can be observed in the symptoms of bacterial disease (Figure 4D, E).
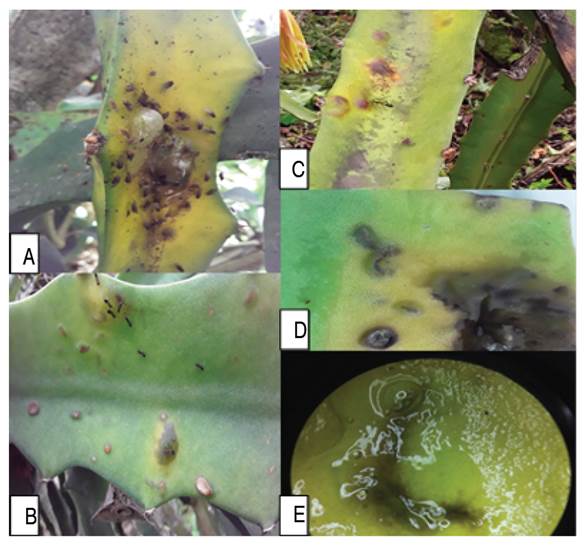
Figure 4 Possible vectors of bacterial disease in pitahaya (Selenicereus megalanthus Haw.). A) Diptera, flower bud fly (Dasiops saltans) are attracted to and feed on mucus or mucilage, and the fluid is one of the symptoms of bacterial disease. B) The ants walk through the chlorotic stains and excretions and feed on the mucilage that is excreted in the symptoms. C) Diptera feeds on the blisters in the early stages of the disease. D-E) Stereoscopic view (10x) of the unidentified larval stage inside the stems during the initial stages of the disease. Small galleries and paths of the larva can be observed in the symptoms of bacterial disease such as spots of chlorosis, blisters, small translucent drops, and mucoid.
For Colombian crops, the stink bug Leptoglossus zonatus (Dallas) (Hemiptera: Coreidae) and the pitahaya's flower bud fland Dasiops saltans Townsend (Diptera: Lonchaeidae) have been identified as possible vectors of the disease or it can be insects that cause wounds that facilitate the infection by phytopathogens (Burgos 2013). Likewise, the fly Neosilba could be a vector of basal rot of the fruit, and for future publication in this study Fusarium oxysporum was detected in basal rot of stem (unpublished data).
Two types of morphotypes were isolated of the leg's insect Leptoglossus zonatus. By molecular technique, the species Pseudomonas fulva and Lysinibacillus fusiformis were identified. Associated insects of the Order Diptera, flies belonging to the family Lonchaeidae, and ants of the family Formicidae have been identified in pitahaya crops in Colombia, with a positive correlation of the number of insects with the number of cladodes and the number of fruits of yellow pitahaya, adapting to microclimates thanks to the forms of the plant. Therefore, proper pruning must be maintained, removal of diseased material and avoid leaving open wounds on the stems of the plants as a prevention strategy and phytosanitary management, especially in periods of high precipitation (Medina and Kondo 2012; González-Trujillo et al. 2019).
CONCLUSION
This study describes the etiology and bacteria associated with the symptoms of soft rot of pitahaya stems from Boyacá, Colombia. Twenty-five bacterial morphotypes were isolated, identified and grouped into 13 genera. Most bacterial isolates belong to the order Enterobacterales suggesting a possible complex of bacteria in the phytopathogenic system. Within the group of Enterobacteria, Kluyvera, Enterobacter, Klebsiella, Pantoea, Pectobacterium and Erwinia were identified. This is the first report in Colombia of Enterobacter cloacae, Pectobacterium carotovora and Paenibacillus glucanolyticus, associated with the soft rot of the pitahaya stem and as potential phytopathogens in pitahaya, since the pathogenicity tests did not obtain positive results for in vitro infection. The genera Achromobacter, Pseudomonas, Klebsiella, Stenotrophomonas, Microbacterium, and Sphingobacterium were identified by molecular techniques like the microbiota associated with stem symptoms, possibly as endophytes, necrotrophic bacteria, or commensal bacteria. The etiology of the disease is also described in the field, identifying the signs and symptoms such as small chlorosis of the stems, and it can spread throughout the entire area of the cactus pads, to later generate softening of the stem, dark brown coloration with odors of bacterial rot. Different management practices such as preventive pruning, the use of microbiological control agent and organic management of the farm, cleaning, and disinfection of material as a cultural control practice could work to control the disease. The yellow pitahaya constitutes an exotic, "orphan" crop and valuable crop chain that justifies research and innovation investment to assure competitiveness and socio-cultural and environmental preservation values. Likewise, the identification of the phytopathogens and the preservation of the strains allow the study of chemical and biological control agents as an alternative in the management of the disease.