Currently, the use of legumes as living soil covers has proved to be a socially, economically, and environmentally viable alternative for integrated soil management, due to its interrelation with atmospheric nitrogen-fixing bacteria (Bécquer and Prévost 2016). In addition, they conserve soil moisture, improve water absorption, reduce erosion by runoff or leaching, and increase soil conservation. They also suppress the weed population and reduce the spread of pathogens (Labrada 2004).
Conventionally, current agricultural systems eliminate the weeds that grow in crops and most of the time the soil remains completely open and exposed to environmental factors (sun, wind, rain, etc.) (Cotler et al. 2020) causing to a greater or lesser extent fertility loss and erosion, which involves the detachment, transport, and deposition of soil particles due to water or wind; it is a natural process that depends on climate, soil type, topography, and vegetation (García 2016). In response to these problems, sustainable alternatives have emerged such as agroforestry techniques, crop rotation, and the use of covers, among others. Their purpose is to avoid soil degradation and increase yields.
Regarding the use of covers, Neonotonia wightii has established itself as one of the main plants of the Fabaceae family. Because of its African origin, it can withstand periods of drought, and due to its perennial, voluble, and branched habit it contributes to weed control in subtropical and tropical perennial fruit crops (Jannoyer et al. 2011; Morris et al. 2013). This makes it an environmentally viable strategy for the control of weed populations.
However, its conventional propagation method (by seed) has inhibited its establishment in the field due to the presence of an impermeable seed coat that acts as a physical barrier and limits the diffusion of water and gases into the seed, which prevents a uniform development and germination of embryos in a shorter period of time even when the conditions are favorable (physical latency) (Acosta et al. 2020).
Given the particular nature of this seed, the effectiveness of various chemical and mechanical scarification methods has been tested. Their purpose was to break the impermeable layer that surrounds it (Baskin and Baskin 2014) and increase the percentage of germination. The treatments used include sulfuric acid, sandpaper, and hot water, among others (De Morais et al. 2014).
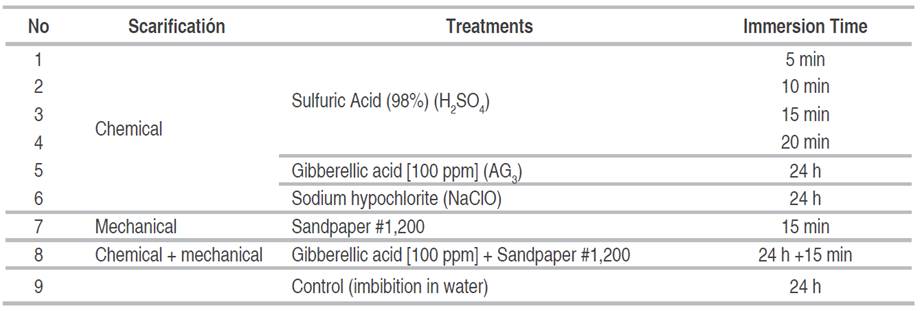
This study aim was to determine which scarification method stimulates the germination process to a greater extent. Different immersion times were evaluated, from 5 to 20 minutes in sulfuric acid, 24 hours in sodium hypochlorite, water and gibberellic acid, 15 minutes of treatment with sandpaper, and a combination of the last two.
MATERIALS AND METHODS
Study area
The study was conducted at the Fruit Crop Nutrition laboratory at Chapingo Autonomous University, Mexico, in the summer of 2022.
Plant material
Forage soybean seeds (Neonotonia wightii var. Cooper) obtained from dry pods containing 5-6 dark brown seeds, selected from random plants from the municipality of Álamo Temapache-Veracruz, Mexico, were used; located between parallels 20°47' and 21°12' N, meridians 97°30' and 97°56' W, and at an altitude between 10 to 500 meters above sea level (masl), in the spring of 2022.
Experimental design
A germination assay was performed where the treatments described in Table 1 were arranged in a completely randomized design with three replicates, using 200 seeds per experimental unit.
Management of the experiment
Seeds that were treated with chemical scarification methods were washed with abundant distilled water, subsequently, 100 were placed in each Petri dish (diameter 100 mm) with moist filter paper, kept in darkness for 2 days at room temperature and moistened once a day. Afterwards, they were kept in germination chambers (SEEDBURO brand) at a constant temperature of 27±2 °C and moistened daily with distilled water by means of a syringe (from 3 to 13 mL as the seeds germinated) during the runtime of the assay.
Evaluated variables
Seed germination was visually assessed every 2 days, until 15 days after sowing. Through daily observations it was possible to evaluate the variables that make up the germination process such as:
Germination percentage (GP): Number of germinated seeds/total seeds, expressed as a percentage (%). A seed is considered germinated when the radicle emerges from the testa (Maqueira-López et al. 2021).
Mean germination speed (MGS): expressed in seeds germinated per day (González-Amaya et al. 2018).
Mean germination time (MGT): considering the days needed for 50% of the total of germinated seeds to germinate (González-Amaya et al. 2018).
Data analysis
After the assessments, the germination percentage (GP), mean germination speed (MGS) and mean germination time (MGT) were calculated. The response variables of GP and MGS were square root-transformed because the data were not normally distributed according to the Shapiro-Wilk test (P≤0.05). An ANOVA and Tukey's multiple-means comparison test were performed, with a 5% probability of error using the statistical program Infostat version 2020 (Di Rienzo et al. 2020).
RESULTS AND DISCUSSION
Analyses were performed for data collected over a 15-day period. Mean comparisons showed significant differences for the variables under study, except for Mean Germination Time (MGT), indicating differential responses of soybean seeds to each pre-germination treatment (Table 2).
Table 2 Mean values (± standard error) and Tukey's mean comparison for the variables germination in soybean seeds subjected to different scarification methods.
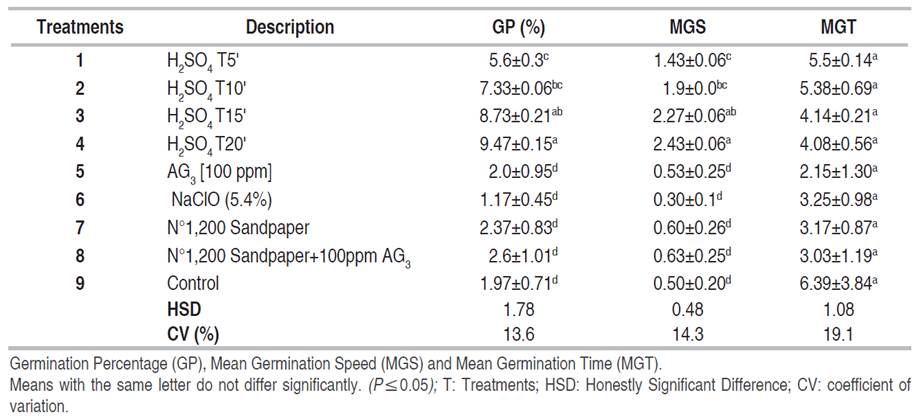
The 20 min immersion time in sulfuric acid (T4) was statistically superior in the variables Percentage and Mean Germination Speed with respect to the control and the seeds subjected to sodium hypochlorite, sandpaper #1200, gibberellic acid and the combination of the latter two.
However, in terms of Mean Germination Time (MGT) there were no significant differences between treatments. It should be noted that there were no significant differences between the immersion times (10, 15 and 20 min), but there were significant differences with respect to T1 (H2SO4T5').
These differences can be explained by the fact that, by not altering the seed coat as occurred in the control treatment, the embryo failed to activate, thereby inhibiting germination. This happens because the embryo is enclosed within an impermeable cover (Varela and Arana 2011) that prevents the passage of water (called physical latency). When it gets damaged by artificial methods of chemical or mechanical scarification, which weaken or break the teguments, it allows greater water entry and gas exchange, facilitating the expansion of the embryo and the exit of the radicle.
This indicates that, as the seed's exposure time to sulfuric acid increases, the seminal coat or testa is weakened by the corrosive action of the acid, resulting in an increase in the permeability of the seed coat, and therefore in the proportion of the variable GP, going from 1.17 in sodium hypochlorite to 7.3, 8.73 and almost 9.47 in T2, T3 and T4, respectively, exceeding those reported by Febles and Padilla (1977), who immersed Neonotonia wightii seeds in concentrated sulfuric acid for 60 min and obtained a 31% germination rate, while in the control it was only 1%. Another study reported a 50% germination rate in seeds immersed in the same acid at 98% for 30 min, compared to zero percent in seeds that were in hot water immersion at 70 and 80 °C for 10 and 15 min, respectively (Tauro et al. 2009). On the other hand, Lima et al. (2019) found that there were no significant differences in seed germination of three forage species (Avena strigosa Schreb, Calopogonium mucunoides, and Neonotonia wightii, all collected from animal feces at intervals of 6 up to 48 h after ingestion, and where only 5% of germinated seeds were obtained in Neonotonia.
The results with another legume species (Amburana cearensis) showed that immersion in sulfuric acid for 10 min stimulated germination, resulting in a higher proportion of PG (0.9 to 1) compared to other treatments (immersion in distilled water at 40, 60, 80, and 100 °C for 2 min) (0.2 to 0.6) (Galíndez et al. 2015).
The presence of an impermeable seed coat (generating physical latency) is typical of some forage legumes (Camacho 2011). Therefore, when the testa is partially or totally damaged, it is easier for water to pass through these tissues, activating enzymes and protein synthesis, and hydrolyzing starches, lipids and endosperm proteins into sugars, fatty acids, and amino acids, necessary at the growing points of the embryonic axis for cell expansion and mitotic division, until the radicle and plumule appear (Finch-Savage and Leubner-Metzger 2006). Thus, acid immersion time is a key factor in stimulating germination in seeds with physical latency, since not all seeds have a favorable response to acid immersion.
Due to the scarcity of updated information on germination tests with sulfuric acid and immersion times and other scarification methods in this family, the decision was made to compare with other species. Merino-Valdés et al. (2018) found that, in seeds of Capsicum pubescens, the presence of H2SO4 at concentrations of 100, 75 and 60% with an immersion time of 30 min, had a negative effect on germination, it caused the seminal coat to rupture, which lead to the destruction of the embryo.
The seeds that were treated with #1200 sandpaper were statistically equal to the control in the GP variable, but inferior to those subjected to sulfuric acid, including those reported by Flores et al. (2020), where a 90% germination rate was obtained using an equipment set at 1,330 rpm with #60 sandpaper for 2 min and friction for 5 min with #125 sandpaper, respectively.
Similarly, for treatments T5, T6 and T8, the rates were significantly lower. Castillo-Quiroz et al. (2018) found that immersion in 3% NaClO for 8 min favored germination in Nolina cespitifera, which, like soybean seeds, is characterized by physical latency, obtaining 49.3%, indicating that NaClO is indeed a germination-inducing agent in this species. The longer exposure time (24 h) and higher concentration (5.4%) may have had a negative effect on the germination percentage, as the result in this study was lower than the one reported by this author.
In the case of gibberellic acid, which is characterized as a phyto-regulatory hormone that is directly involved in the control and promotion of seed germination, it can break seed latency and replace the need for environmental stimuli, such as light and temperature (Araya et al. 2000). Saldívar-Iglesias et al. (2010) found that by applying 250 mg L-1 of this acid, they obtained an -87% germination rate of Jaltomata procumbens (Cav.) J. L. Gentry, which is higher than what was reported in this study, as the concentration used here was lower (100 mg L-1).
As a result, the slowest germination speeds (germinated seeds per day) occurred in these treatments compared to seeds that were subjected to different immersion times with 98% sulfuric acid (5, 10, 15 and 20 min).
CONCLUSION
The presence of an impermeable coating inhibits germination in soybean seeds. This was determined as treatments removed the coating. Therefore, immersion in sulfuric acid for different periods of time was more effective than the rest of the treatments, favoring the germination process of the seeds. However, it was not possible to conclude or demonstrate in what time it is possible to obtain a 100% germination rate, therefore, it is recommended for future research to continue with this treatment reaching up to 35 min of immersion in this acid.