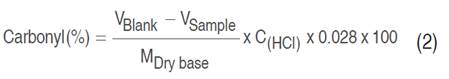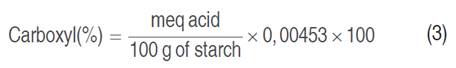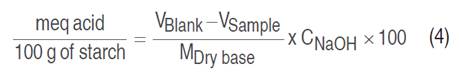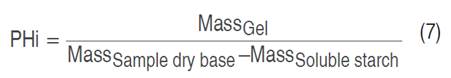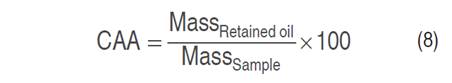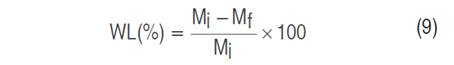There are new packaging methods, among which, coatings are qualified as a promising technology due to their applicability and characteristics attributed to the different products involved in their matrix. Besides, these coatings are environmentally friendly, as they try to minimize the impact of traditional packaging by using natural and biodegradable biopolymers that are obtained from natural sources or extracted from industrial by-products (Fernández et al. 2017).
The coatings consist in coating a fruit or vegetable with one or several thin layers of a continuous matrix that fits around the product. They are usually applied by immersion in the coating-forming solution, to reduce quality loss and extend the shelf life of fruits and vegetables, either whole or minimally processed, in synergy with other treatments and ingredients (Fernández et al. 2017).
Among the polysaccharides of greatest interest for the formulation of coatings is starch, which forms cohesive molecular networks that confer good mechanical properties such as adhesion and flexibility, which depend on the composition and source of the starch. These coatings are tasteless, odorless, and transparent, having a minor impact on the coated product. However, they present poor barrier properties against water vapor, and their semi-crystallinity and rapid degradation affect their mechanical properties, so their structure must be modified to improve these properties (Ramos-García et al. 2018).
Oxidation is a feasible chemical modification route, in which sodium hypochlorite (NaOCl) acts as an oxidizing agent. This reaction consists of the introduction of carbonyl and carboxyl groups that hydrate and swell the molecule better, improve stability at high temperatures, and impart transparency and low viscosity compared to native starch. During starch oxidation, the primary hydroxyl groups are oxidized to carboxyl groups or aldehydes, depending on the conditions of oxidant concentration, pH, and starch origin, among others. Partial depolymerization of the polysaccharide chains also occurs and various degrees of oxidation are obtained; for example, when there is a low concentration of active chlorine in an alkaline medium, a greater number of carboxyl groups are formed (Bonilla et al. 2013; Ramos-García et al. 2018).
Starch oxidation is used in various applications due to its ability to enhance the properties of starch-based materials. Oxidation of starch modifies its chemical structure, improving its solubility and reducing its viscosity, which facilitates the formation of thin, uniform films and coatings. This modification is crucial in the food industry for creating effective edible coatings that improve food preservation. Oxidized starches provide better moisture and oxygen barrier properties, which are essential for extending the shelf life of perishable products. Studies have shown that oxidized starch coatings can reduce moisture loss and delay spoilage, which contributes to better preservation of fruits and vegetables, such as cherry tomatoes (Zamudio-Flores et al. 2010). Furthermore, these coatings often exhibit antimicrobial properties, which help in controlling microbial growth and reducing fungal decay, thereby enhancing the shelf life and safety of the food (Ungureanu et al. 2023). Overall, the use of oxidized starch in edible coatings is justified by its improved functional properties and effectiveness in food preservation.
Among these products, there is the cherry tomato (Solanum lycopersicum cv. Cerasiforme). The cherry tomato is a vegetable of importance due to its wide consumption, either as a fresh product or as raw material for the food industry and is characterized by a short shelf life (Pérez-Díaz et al. 2020); due to physiological changes during its postharvest stage. Its aw is 0.94, which is associated with the loss of water vapor during transpiration (Fich et al. 2020) and the subsequent weight loss due to dehydration. This, in turn, causes softening, flaccidity, and loss of turgidity and texture.
Previous studies have shown that edible coatings enhance the quality and shelf life of cherry tomatoes. For instance, the application of cornstarch coatings on tomato fruits has proven effective in maintaining postharvest quality by reducing weight loss and delaying ripening (Fitch-Vargas et al. 2019). Other studies have highlighted the use of various starch modifications, including oxidized and heat-moisture-treated starches, which enhance the mechanical and barrier properties of the coatings (Zavareze et al. 2012).
Considering the above, this research aimed to evaluate the influence of a coating based on banana (Musa paradisiaca L. AAA group, cv. Cavendish) oxidized starch and olive oil on the preservation of cherry tomatoes (Solanum lycopersicum cv. Cerasiforme) during their storage at ambient conditions.
MATERIALS AND METHODS
Isolation and chemical modification of banana starch
Green bananas (Musa paradisiaca L. AAA Group, cv. 'Cavendish') with a maturity grade of one according to the Von Loesecke scale and caliber of 39 to 42 cm (NTE INEN 2801, 2013), were acquired in the twelfth week of harvest, in a farm in La Iberia parish of El Guabo canton of El Oro province, Ecuador.
Starch isolation was carried out by the wet method (Hidalgo et al. 2020). For this, once the peel was removed, the fruit was cut into pieces 2 to 3 cm long. Wet grinding was achieved with a 3% (m v-1) citric acid solution at a ratio of 1:0.25 with a domestic blender at 3,600 rpm for 2 min. The product obtained was filtered through a 345 µm mesh and washed until the outlet liquid was free of apparent starch residues. Subsequently, the liquid was allowed to settle for 3 h. The sediment was centrifuged (Corning® LSETM, NY, USA) at 5,000 rpm and the supernatant was removed. The paste (native starch) was centrifuged again and oven-dried (Memmert, Schwabach, Germany) at 50 °C for 24 h to a constant mass. Finally, the dried product was ground and passed through a No. 60 mesh sieve (250 µm, ASTM E11, Gilson Co. Inc., USA) to obtain a uniform particle size. The yield (%) was calculated using Equation 1, Both masses were expressed in g.
Starch oxidation
Starch oxidation was done according to the methodology proposed by Carhuallay et al. (2020) with some modifications. A starch solution (100 mL) was prepared at 30% (m v-1); this was then placed in a thermo-stirring plate (Cimarec+TM Thermo Fisher Scientific Inc., Waltham, Massachusetts, USA) at 525 min-1 and heated to 30 °C. The pH was then adjusted to between 9 and 9.5 with NaOH at 2 N. Next, 100 mL of 1.5% (m v-1) NaClO was added dropwise for 30 min. During this time, the pH was maintained between 9 and 9.5 with H2SO4 at 1 N. The solution was continuously stirred to promote the reaction according to the time evaluated in each treatment (40 and 90 min) and the pH was kept constant with a solution of NaOH at 1 N. When the reaction was finished, 1 g of sodium bisulfite was added and left to act for 1 min. The effluent was filtered with Whatman® qualitative filter paper until a clear liquid was obtained. The retained product was placed in an oven at 45 °C for 16 h until constant mass and was then ground and sieved.
Determination of carbonyl and carboxyl groups of starch
The carbonyl group was determined according to a volumetric method adapted by Fonseca et al. (2015). Four grams on a dry basis of oxidized starch was added to 100 mL of distilled water in a 500 mL beaker. The suspension was gelatinized in a boiling water bath for 20 min. The suspension was cooled until 40 °C and the pH was maintained at 3.2 with 0.1 N HCl. Following this, 15 mL of a hydroxylamine reagent were added to the mixture; the beaker was capped and placed in a water bath at 40 °C for 4 h with slow stirring on a thermostirring iron. For the preparation of the hydroxylamine reagent, a 500 mL volumetric flask was used in which 25 g of hydroxylamine hydrochloride were dissolved in 100 mL of NaOH at 0.5 N, completing the volume with distilled water. The reaction mixture was titrated to pH=3.2 with 0.1 N standardized HCl to determine excess hydroxylamine. The blank was titrated with native starch and hydroxylamine reagent following the same procedure. The carbonyl percentage was calculated according to Equation 2.
Where V is the volume consumed by the blank and sample, expressed in mL; M is the mass in g; and C is, the equivalent molar concentration of HCl.
The carboxyl group was quantified according to Ashwar et al. (2014). In a 100 mL beaker, 2 g (bs) of starch was placed, 25 mL of HCl at 0.1 N was added and continuously stirred in a thermostirring iron at 350 min-1 for 30 min. The suspension was then filtered and washed with sufficient distilled water. The starch was then placed in a 600 mL beaker and 300 mL of distilled water was added; the solution was boiled for 10 min to achieve complete gelatinization and allowed to cool to 40 °C. Subsequently, it was titrated with NaOH at 0.1 N. This procedure was also performed with the native starch as blank. Equations 3 and 4 determined the carboxyl content (%):
Where V is the volume consumed by the blank and sample, expressed in mL; M is the mass in g; and C is the equivalent molar concentration of NaOH.
Proximal composition of starches
The determination of the proximate composition of native and oxidized starches included the determination of protein content by the Dumas method (Mikó et al. 2023), crude fiber by the Weende method (Lopes et al. 2021); lipids and ash according to AOAC (2020). The moisture content was determined according to AOAC (2020). The total carbohydrate content was determined by difference.
Functional characterization of starches
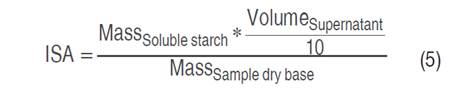
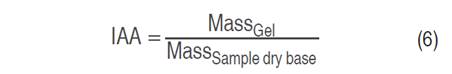
Water solubility index (ISA), water absorption index (IAA), and swelling power (PHi) values were determined (Salcedo-Mendoza et al. 2016). To 1 g (dry basis) of starch contained in a test tube, 25 mL of distilled water preheated to 60 °C was added. The suspension was kept in a water bath at 60 °C for 30 min and manually shaken for 10 min after the heating started. Subsequently, it was centrifuged at 2,500 min-1 for 15 min and the supernatant (soluble starch) was recovered and the total volume was determined. Next, 10 mL of the supernatant was deposited in a Petri dish, previously weighed; it was dried in an oven at 70 °C for 16 h. The masses of the Petri dish with the material and the centrifuge tube containing the gel (insoluble starch) were recorded. The values of ISA, IAA, and Phi were calculated from Equations 5, 6, and 7, respectively.
All masses were expressed in g and volumes in mL.
Oil absorption capacity (CAA) was determined using the methodology proposed by Olatunde et al. (2017) with modifications. In a centrifuge tube, previously weighed, 2 g of sample was deposited and mixed with 20 mL of edible oil. It was allowed to stand at room temperature for 30 min; then it was centrifuged at 2,000 min-1 for 25 min and the supernatant oil was removed, finally, the tubes were weighed on an analytical balance and the results were expressed according to Equation 8.
The CAA was expressed in %, and both masses in g.
For the determination of the gelling temperature, a 10% (m v-1) starch solution was prepared in a 150 mL beaker, which was placed in a water bath at 85 °C. A thermometer was then placed inside the beaker and stirred continuously. Finally, the reading was taken when a paste was formed and the temperature remained stable (Carhuallay et al. 2020).
Selection of a coating based on the weight loss of cherry tomatoes
For the formulation of the coatings, an experimental design 22 completely randomized was applied using the Statgraphics Centurion XVI.I program (Statgraphics Technologies Inc., Virginia, USA), which was executed in three blocks, generating 12 runs that included eight replicates. The oxidized starch content was adjusted to 4% (m v-1) in all runs. The percentages of glycerol (3 and 3.5% mv-1) and olive oil (0.3 and 0.4% m v-1) were considered as design factors. The effect of the treatments on weight losses of cherry tomatoes (S. lycopersicum cv. Cerasiforme) was analyzed and a model was obtained for its estimation under the storage conditions studied. The results were expressed as percentage weight loss (WL) according to Equation 9.
Where WL is the weight loss (%); Mi is the initial mass (g); and Mf, the final mass (g).
Preparation and application of coating-forming emulsions
For the preparation of the coating-forming emulsions, the methodology described by El Halal et al. (2015) was followed with some modifications. Starch with a high degree of oxidation was used to prepare the coating-forming emulsions. The oxidized starch solution (4% m v-1) for each of the treatments was heated with constant stirring to a temperature between 68 and 69 °C, corresponding to the gelatinization temperature (Schmiele et al. 2019). Next, the amount of glycerol was added according to the experimental design, and stirring was continued for 30 min maintaining the temperature constant. After cooling the solution to 35 °C, Tween 80 was added as an emulsifier considering a 1:1 ratio based on the amount of olive oil and stirring was continued for 1 min. Subsequently, the olive oil was added; this dispersion was homogenized (Homogenizer D-500, DLAB Scientific Instrument Inc., California, USA) at 15,000 rpm for 4 min.
To evaluate the effect of each of the coatings, selected cherry tomatoes were used, considering that they all presented similar sizes and ripening degrees of four (Saborío 2021) and without mechanical damage. The tomatoes were disinfected with a NaClO solution at 5 mg kg-1 for 15 min, dried and coated by immersion in the film-forming emulsion for 4 min, and left to dry at ambient conditions for 48 h. The tomatoes were then dried at room conditions for 48 h. The tomato mass was then recorded, and the tomatoes were dried at 5 mg kg-1. The individual mass of the tomatoes was then recorded and considered as the initial mass; the final mass was determined after five days of storage at ambient conditions (20 to 25 °C and 70 to 85% relative humidity), dependent on time of day (Dovale-Rosabal et al. 2015).
Preservation of cherry tomatoes with oxidized starch-based coatings
Based on the results on the effect of coatings on weight loss, a formulation was selected to verify its influence on the preservation of cherry tomatoes during storage under ambient conditions. The selected coating was applied by immersing the tomatoes in the emulsion for 4 min, followed by ambient drying for 48 h (Hoyos-Yela et al. 2019).
During 16 days of storage of cherry tomatoes, refractometric soluble solids (Al-Dairi et al. 2021), titratable acidity (Al-Dairi et al. 2021), pH (NTE INEN-ISO 1842, 2013), and percentage weight loss were determined. To compare the changes, a batch of uncoated tomatoes was kept under the same storage conditions.
Statistical analysis
Analysis of variance was carried out to determine the statistical significance of the results of the different determinations. If there were significant differences, Duncan's multiple range test was applied to determine the differences between means (P≤0.05). These differences were indicated with different letters for a determination.
RESULTS AND DISCUSSION
Yield of banana starch extraction
The banana starch extraction yield was 27.26±3.95%, applying wet milling, determined based on the weight of the peeled immature fruit. This value was similar to those reported by Hidalgo et al. (2020) for plantain (Musa paradisiaca) starch extraction. In these works, the influence of several variables of the wet milling process that affect this indicator, such as grinding speed and time, was explained.
Chemical characteristics of oxidized starch
Table 1 shows the results of the effect of oxidation time on the degree of starch oxidation. An increase (P≤0.05) of carbonyl and carboxyl groups formed was evident as the reaction time at constant temperature increased.
Table 1 Effect of oxidation time on the oxidation degree of banana starch (Musa paradisiaca L. AAA group, cv. Cavendish).
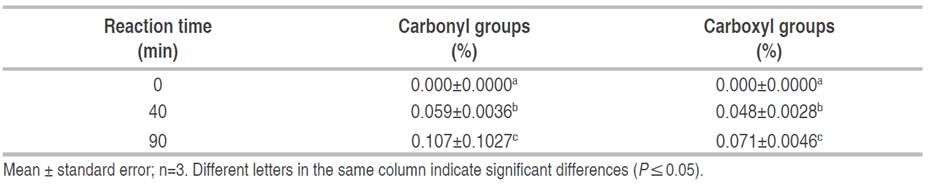
The reaction time of 90 min was more effective in obtaining a higher degree of oxidation, with values of 0.107±0.1027% for carbonyl groups (C=O) and 0.071±0.0046% for carboxyl groups (COOH), behavior similar to those described by Bonilla et al. (2013) and Sumardiono et al. (2021) for cassava and sago starch, respectively. This is related to the oxidizing agent initially converting hydroxyl groups to carbonyl groups (C=O). Subsequently, these are converted to carboxyl groups (COOH) that are selectively formed by the oxidation of hydroxyl groups on the carbons of positions 2, 3, and 6 of the glucose units that make up the starch molecule (Bonilla et al. 2013), defining the degree of substitution. Other factors such as pH, concentration of the oxidizing agent, and temperature influence this reaction.
Effect of oxidation on the proximal composition of banana starch
The protein and moisture contents of the native starch were 2.06±0.0306 and 10.22±0.0833%, respectively (Table 2), values close to those reported by Zamudio-Flores et al. (2015). For lipid, crude fiber, and carbohydrate contents, values of 0.7±0.0379, 0.42±0.0264, and 85.15±0.2685%, respectively, were obtained. These values depend on factors such as the origin and variety of the banana, regional climate, cultural practices, harvesting conditions, isolation methods, and type of oxidizing agent used in extraction, among others (Helen et al. 2022; Olatunde et al. 2017). The ash percentage was 1.46±0.0321%, which could be due to the presence of minerals such as calcium, potassium, and magnesium.
Table 2 Effect of oxidation time on the proximal composition of banana starch (Musa paradisiaca L. AAA group, cv. Cavendish).
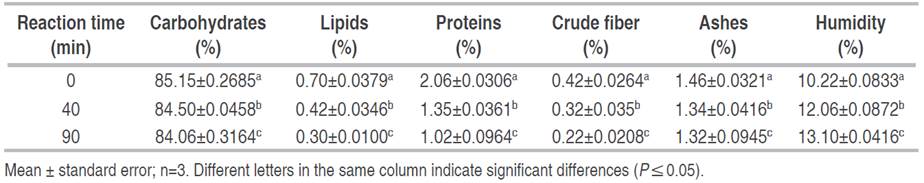
The oxidation reaction time increased the moisture percentage (P≤0.05) in oxidized starches from 12.06±0.0872 to 13.1±0.0416%, compared to native starches. This could have been due to the increase in the hydrophilic potential of oxidized starches because the carbonyl and carboxyl groups inserted in the molecule formed hydrogen bonds with water molecules (Zamudio-Flores et al. 2015). The contents of lipids, crude fiber, proteins, carbohydrates, and ash tended to decrease when the reaction time increased (P≤0.05). This was related to the oxidative effect of chlorine on protein denaturation, lipid saponification, and chemical disintegration in the starch molecule, due to the exposure time to sodium hypochlorite, as well as to the leaching of minerals during the oxidation process (Olatunde et al. 2017).
Effect of oxidation on the functional properties of starch
The technological behavior of starch is in correspondence with its functional properties, which are specific according to the starch origin and degree of modification (Cedeño-Sares et al. 2021). Table 3 shows the values of the functional properties of starches. It is observed that the ISA increased with oxidation time, indicating greater solubility of the oxidized starch. On the other hand, both PHi and IAA decreased, suggesting a lower capacity of oxidized starch to absorb and retain water. CAA increased significantly, demonstrating a better ability of oxidized starch to interact with oils, which would be beneficial in some food applications. The gelation temperature decreased with oxidation time, implying that oxidized starch requires less energy to form a gel, an advantageous characteristic for certain industrial processes.
Table 3 Effect of oxidation time on functional properties of banana starch (Musa paradisiaca L. AAA group, cv. Cavendish).
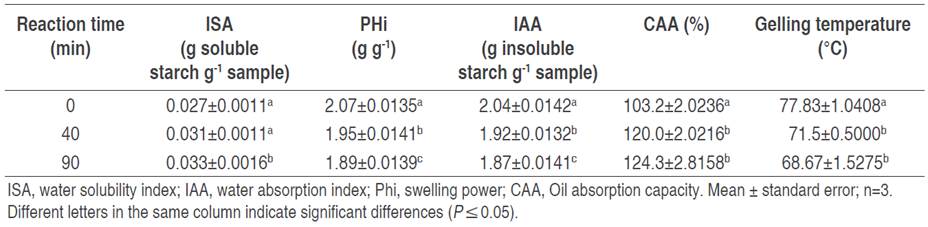
For native starch, these values were similar to those reported by Cedeño-Sares et al. (2021) and Correa et al. (2017) for starch from banana (Musa AAA cv. Cavendish) and Dominican plantain (M. paradisiaca), respectively. However, it was evidenced that increasing the reaction time, increased the ISA values (P≤0.05), expressed as soluble starch, from 0.031±0.0011 to 0.033±0.0016 g g-1 sample, compared to that obtained for native starch (0.027 g g-1 sample). Olatunde et al. (2017) also obtained similar behavior in a study developed with bananas. According to Salcedo-Mendoza et al. (2018), ISA is related to the granule size and amylose content present in starch.
A contrary situation was observed for PHi and IAA values. As the reaction time increased, these values tended to decrease (P≤0.05), compared to those of native starch. This could be due to the structural disintegration of the granules, which would cause a decrease in the amorphous region (Carhuallay et al. 2020), with a low capacity to retain water, decreasing its PHi and IAA, with an increase in solubility (Vanier et al. 2017).
Reaction time increased AAC, coinciding with that reported by De Barros et al. (2016). Among the factors related to this increase, is the presence of crystalline and amorphous regions that are affected by the oxidizing agent (Tovar-Benítez et al. 2019). The native starch presented higher water and oil retention, but lower solubility because its structure remained intact.
Concerning the gelation temperature, the values obtained for native starch corresponded to those reported by Martínez et al. (2015). However, extending the reaction time decreased (P≤0.05) the gelation temperature in oxidized starches. This was due to the early weakening and breaking of amylopectin double helix bonds and the introduction of negatively charged carbonyl and carboxyl groups, facilitating hydration, which would weaken the starch granule and cause it to gelatinize at a lower temperature (Carhuallay et al. 2020).
Influence of coatings on weight losses of cherry tomatoes
From the analysis of weight losses in cherry tomatoes for each of the treatments (Table 4), it was evidenced through the Pareto diagram (Figure 1A), that the percentage of olive oil and its interaction with the percentage of glycerol as components of the coating-forming emulsions, had an impact (P≤0.05) on weight losses, evidencing a correct functionality of the coating. Glycerol is a hydrophilic molecule that facilitates water vapor migration; fatty acids in the film matrix, on the other hand, impart hydrophobicity.
Table 4 Weight loss percentage of cherry tomatoes (Solanum lycopersicum cv. Cerasiforme) in each treatment.
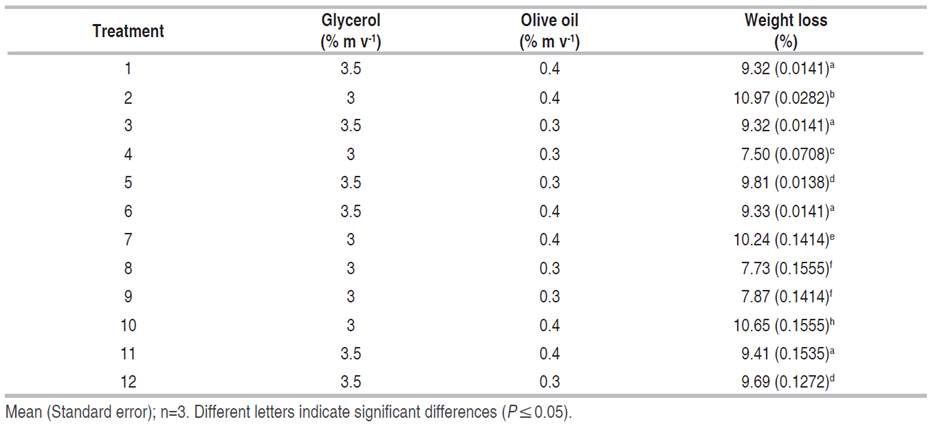
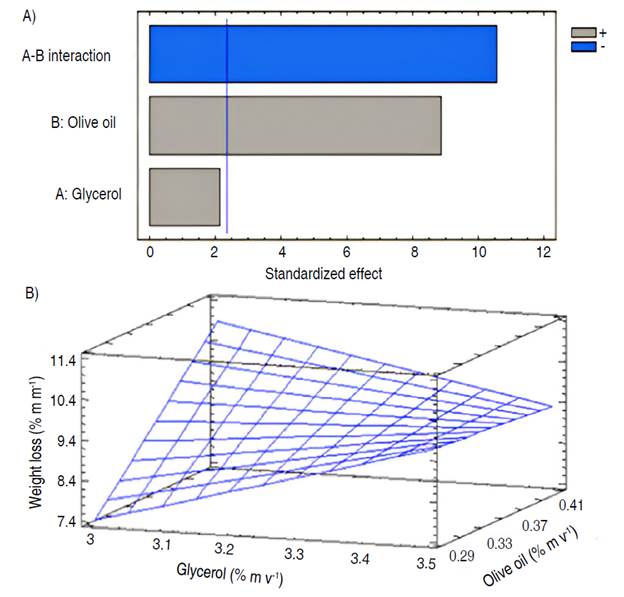
Figure 1 Influence of coatings based on banana oxidized starch and olive oil on weight losses of cherry tomatoes (Solanum lycopersicum cv. Cerasiforme): A) Pareto diagram; and B) estimated response surface.
The estimated response surface diagram (Figure 1B) shows the relationship between the factors studied and their combined effect on weight loss, in correspondence with the adjusted model that describes the behavior of this variable. Treatments with lower concentrations of olive oil (0.3% m v-1) generally exhibit slightly lower weight loss percentages compared to treatments with higher olive oil concentrations (0.4% m v-1), independently of the glycerol concentrations. This could be related to the influence of olive oil on the mechanical behavior of the coating, that is, the concentration of olive oil could affect its physical properties and increase the rate of weight loss during storage (Dovale-Rosabal et al. 2015). The variability in weight loss percentages across treatments indicates that factors other than olive oil concentration, such as glycerol concentration and other experimental conditions, may also influence weight loss in cherry tomatoes.
The value of the coefficient of determination indicated that the adjusted model (Equation 10) was able to explain 96.52% of the variability in weight losses in coated cherry tomatoes, which allowed obtaining the best combination in the experimental region studied, for the formulation of a coating that would minimize weight losses during storage.
Where y is the weight loss; X1 is glycerol concentration; and X2 is olive oil concentration.
The olive oil concentration and its interaction with the glycerol concentration were significant (P≤0.05). Thus, it was obtained that for four oxidized starch coatings, it was necessary to add 0.3% (m v-1) olive oil and 3% (m v-1) glycerol. This formulation corresponded to the coating selected to evaluate its influence on the preservation of cherry tomatoes.
Influence of oxidized starch-based coatings on the preservation of cherry tomatoes
The pH and acidity values presented a characteristic behavior of the ripening of cherry tomatoes during storage (Figure 2), without significant variations due to the coating. The increase in pH and decrease in acidity could be mainly due to the transformation of organic acids into sugars through cellular respiration (Hoyos-Yela et al. 2019), a process that consumes these acids and generates less acidic products, which could raise the pH of the fruit. Furthermore, water loss through transpiration and specific storage conditions, such as the use of the coating, can influence the enzymatic and metabolic activity of the product, facilitating the degradation of organic acids.
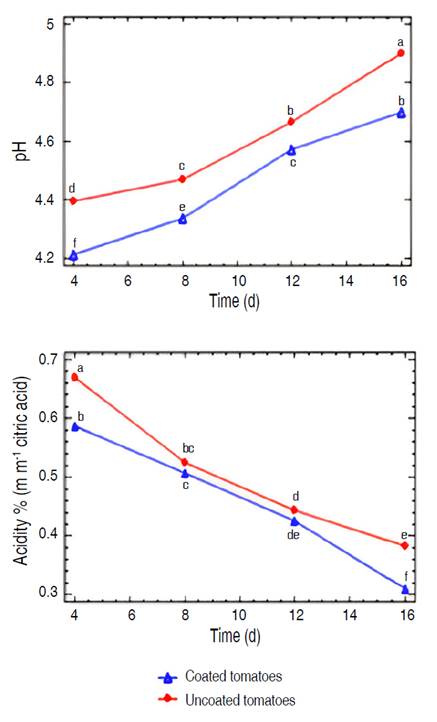
Figure 2 Evolution of pH and acidity in coated and uncoated cherry tomatoes (Solanum lycopersicum cv. Cerasiforme) during their storage at ambient conditions. Different letters indicate significant differences (P≤0.05).
Figure 3 shows the gradual increase in soluble solids associated with the ripening process of cherry tomatoes with and without coating during storage. However, tomatoes with coating presented a lower rate of increase in soluble solids up to 12 days of storage, compared to tomatoes without coating, possibly due to the reduction in respiration and ethylene production (Kumar and Saini 2021). The coatings form a physical barrier that limits the exchange of gases, including oxygen and carbon dioxide. This decreases the respiration rate of the tomatoes and slows down the ripening process and therefore the increase in soluble solids. Additionally, coatings, by reducing ethylene production, delay the conversion of starches to simple sugars, one of the main causes of the increase in soluble solids in tomatoes during ripening.
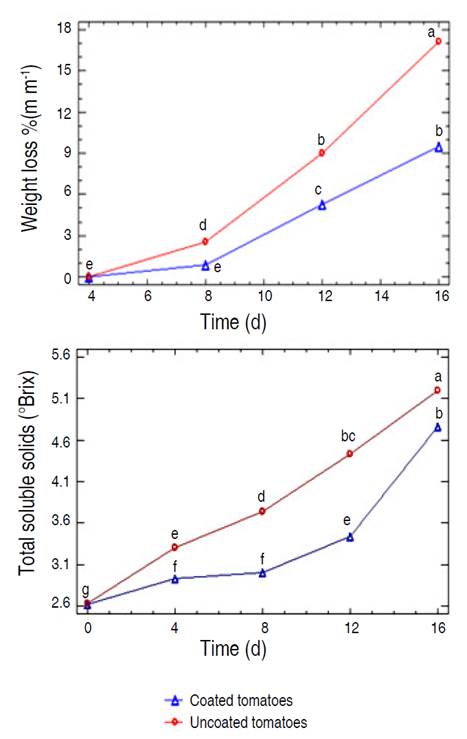
Figure 3 Evolution of weight loss and total soluble solids in coated and uncoated cherry tomatoes (Solanum lycopersicum cv. Cerasiforme) during their storage at ambient conditions. Different letters indicate significant differences (P≤0.05).
The percentage of weight losses increased during storage (Figure 3); however, lower values were recorded for coated tomatoes. Osae et al. (2022) mentioned that coatings act as a semi-permeable barrier against gas and moisture exchange, due to the hydrophobic character of the colloidal matrix, in this case, influenced by the addition of olive oil. Finally, it was evidenced that the coating reduced weight losses in cherry tomatoes by approximately 10%.
CONCLUSION
The research determined the best combination (4% m v-1 oxidized starch coatings, 3% m v-1 of glycerol, and 0.3% m v-1 of olive oil), for the formulation for a coating based on oxidized banana starch and olive oil, offering valuable information on food preservation techniques. It demonstrated the potential of this coating to reduce weight loss and delay the ripening of cherry tomatoes during storage. By elucidating the effects of reaction time on the properties of oxidized starch and identifying the best coating composition, the study contributes to the understanding of starch-based coatings for food preservation. The extraction yield of banana starch was 27.26% by the wet milling method. Lipid, crude fiber, protein, carbohydrate, and ash contents tended to decrease in oxidized starches as reaction time increased, the opposite behavior for moisture content (P≤0.05). Solubility and oil absorption capacity increased in oxidized starches, while water absorption and swelling power decreased (P≤0.05). With the application of the coating, weight losses were reduced in cherry tomatoes stored for 16 days under ambient conditions, and lower soluble solids values were obtained, as an indicator of the delay in the ripening process. These results support sustainable food packaging solutions by utilizing natural materials such as banana starch and olive oil, potentially reducing reliance on synthetic additives. Additional research could explore the long-term effectiveness and scalability of the developed coating under storage conditions. Comparative studies with other preservation methods or coatings could provide information on the relative effectiveness and applicability of this technique.