INTRODUCTION
The Ericaceae family includes the Vaccinium genus, which has approximately 450 species worldwide, distributed mainly in the northern hemisphere (Lim, 2012). The blueberry is one of these species (Vaccinium corymbosum L.), is native to the United States, and is the most important species cultivated for commercial and industrial purposes because of its size and variety of antioxidants in the fruits (Miao et al., 2022). These properties have generated great interest, especially in the nutritional pharmaceutical industry where it is known as a "super fruit" that is used in the treatment of various diseases (You et al., 2011; Stevenson and Scalzo, 2012). In Colombia, the departments of Cundinamarca and Boyaca are the larger producers, where all production is used to satisfy local consumption (Fernández-Vargas et al., 2020). Therefore, blueberry cultivation represents an opportunity for continuous production that could meet domestic demand and help meet the needs of countries in the northern hemisphere in the winter months (Cortés-Rojas et al., 2016).
However, blueberry production can be affected by the shallow and highly branched root system, which has little or no root hairs, with consequent restrictions on the ability of plants to absorb water and nutrients (Kim et al., 2011). This can affect the synthesis of photosynthetic pigments such as chlorophylls (Yu et al., 2015).
The mineral nutrition of crops is of great concerns for producers. Diagnostic tools such as soil or tissue analysis are currently used, which are expensive and time-consuming, limiting rapid decision-making (Güiza-Castillo et al., 2020). Destructive methodologies are used to quantify photosynthetic pigments that, while being important in the evaluation of the physiological status of plants, are expensive and not easily accessible for producers (Amarante et al., 2009).
The chlorophyll content in leaves is one of the more important variables when evaluating the physiological status of plants (Amarante et al., 2009). The leaf chlorophyll concentration is generally determined by extracting leaf samples, with subsequent spectrophotometric measurements (Porra et al., 1989; Parry et al., 2014). Such determinations are destructive, costly and time consuming and, therefore, may not be practical. There are also methods that use measurement equipment, which estimate the chlorophyll concentration index quickly, non-destructively, and directly in the field (Güiza-Castillo et al., 2020). These methods exploit the optical properties of leaves and are based on the reflectance and/or absorbance of radiation by chlorophyll (Uddling et al., 2007).
In recent years, several technological solutions have been made available for real-time detection of the concentration of some photosynthetic pigments, such as chlorophylls and their relationship with nutrients such as nitrogen, improving plant management and nutritional values in crops (Edalat et al., 2019). Direct chlorophyll measurement equipment is generally easy to use, does not require any specific user training, and takes measurements quickly with little data processing (Gianquinto et al., 2006). However, given the relatively small sampling area, strict sampling protocols and calibration curves between readings and the chlorophyll concentration in tissue samples must be developed for practical uses (Netto et al., 2005; Padilla et al., 2019).
Measuring the chlorophyll index Soil Plant Analysis Development (SPAD) (Ghosh et al., 2020) has been increasing in agriculture because it provides a fast, simple and convenient method to non-destructively measure the chlorophyll content of crops and leaves (Li et al., 2019; Zhang et al., 2020; Aldana et al., 2014). In addition to their ease, small size, and relatively low cost, chlorophyll meters are well-suited for the field because they provide rapid assessments of chlorophyll concentrations in different crops (Padilla et al., 2018). These benefits have created much interest in the rapid analysis of the total chlorophyll index (Mehrabi and Sepaskhah, 2022).
The SPAD has been widely used to directly determine the chlorophyll index, which has been correlated with elements such as nitrogen and magnesium in the leaves of different plant species, such as grapes (Castañeda et al., 2018; Ates and Kaya, 2021), guava (Güiza-Castillo et al., 2020), soybean (Yokoyama et al., 2018), and wheat (Mehrabi and Sepaskhah, 2022). Therefore, the present study aimed to estimate the concentrations of chlorophylls a, b and total in blueberry leaves (Vaccinium corymbosum L.) from the Biloxi, Legacy and Victoria c using the spectrophotometric method to observe the correlation with the non-destructive SPAD method cultivars under the conditions of the municipality of Paipa (Boyaca).
MATERIALS AND METHODS
This study was carried out on the Tunguavita Experimental Farm of the Universidad Pedagógica y Tecnológica de Colombia (UPTC) in the municipality of Paipa (Boyaca, Colombia), with the coordinates 5°45' N and 73°45' W, at 2,525 m a.s.l., with an average temperature of 15.5°C and average monthly rainfall of 80.5 mm according to the weather station located on the farm during this research, which was carried out during the first semester of 2022.
Biloxi cultivar plants were used, which have medium-sized fruits, a light blue color, high firmness values, and excellent flavor. Legacy is a vigorous and productive cultivar, and its berries are medium-sized and very firm, with good flavor and little scaring (San Martín, 2013). Victoria is a vigorous, late-ripening cultivar with large, easy-to-harvest fruits. The plants were sourced from Fallcreek - Farm & Nursery, Inc. For each cultivar, 30 plants were selected 6 months after transplantation with an approximate height of 45 cm. The plants were planted under crystal-colored anti-hail mesh with a shading percentage of 3% according to the manufacturer's technical information, in 60 L bags with rice husks, pine shavings and black soil substrate. The plants were placed 0.45 m apart, with 1.4 m between rows. To guarantee a weekly water supply of 0.8 L/plant, a drip irrigation system was employed with a crown arrangement using 4 drippers of 1 L h-1. Nutrition was managed following the recommendation of Hirzel (2013), adding in kg 60 of N, 40 of P, 70 of K, 14 of Ca, 8 of Mg, and 8 of S. The micronutrient source guaranteed in kg 0.2 of B and 0.3 of Zn, distributed in a cycle of 52 weeks.
To quantify the total chlorophyll index (SPAD), 30 plants were selected, 10 leaves were taken from the branches in the middle-third that were fully-expanded, and measurements were taken at 10 a.m. to avoid photoinhibition and leaves with low photosynthetic activity according to Deok Han et al. (2022a) and Deok Han et al. (2022b). The measurements were taken with a SPAD-502® (Konica Minolta Camera Co., Osaka, Japan), reporting the average for each plant.
The contents of chlorophylls a (chl a) and b (chl b) was determined with spectrophotometry using a Thermo Scientific™ Genesys™ 10 UV spectrophotometer, following the methodology described by Solarte et al. (2010): taking leaf circles with a radius of 0.5 cm from the middle part of the leaves (between the central rib and the margin), which were macerated to 0.5 g, and adding 14 mL of 80% acetone (v/v), previously cooled at -10 ºC for 5 min, followed by vortex for 2 min and centrifugation for 10 min at 4,000 rpm. The supernatant was removed to a 25 mL volumetric flask, which was covered with paper aluminum, with previously-cooled 80% acetone (v/v). The entire process was carried out with low illumination, taking absorbance readings at wavelengths of 663 nm for chl a and 647 nm for chl b. The analytical blank was 80% acetone (v/v). The contents of chl a, b and a+b were calculated according to Lichtenthaler (1987) for 80% acetone (v/v) (Equations 1, 2 and 3), expressed in mg g-1 fresh weight (FW).
Statistical analysis
The data obtained for chl a, b and a+b were correlated with the total chlorophyll index (SPAD) using the Pearson correlation (r), evaluated with the Shapiro-Wilk test (P<0.05). Subsequently, simple linear regression was performed with the lm function. The correlation analysis was done with the statistical package Corplot from R Core Team (2022).
RESULTS AND DISCUSSION
According to the results of the Pearson correlation analysis, a linear association was observed between the SPAD method and the contents of chl a, b and a+b in the leaves, with positive and significant correlation coefficients in the cultivars and values that ranged between 0.93 and 0.99 (Fig. 1). These results agree with those reported by Güiza-Castillo et al. (2020), who studied guava plants at different phenological stages and observed that the SPAD chlorophyll index presented a positive correlation with the contents of chl a, b and a+b, quantified with spectrophotometry, reporting correlation values greater than 0.91. These results also agree with Lee et al. (2019), who indicated that, when evaluating Arisoo variety apple plants, an r correlation of 0.95 was presented between SPAD readings and chla+bcontent values.
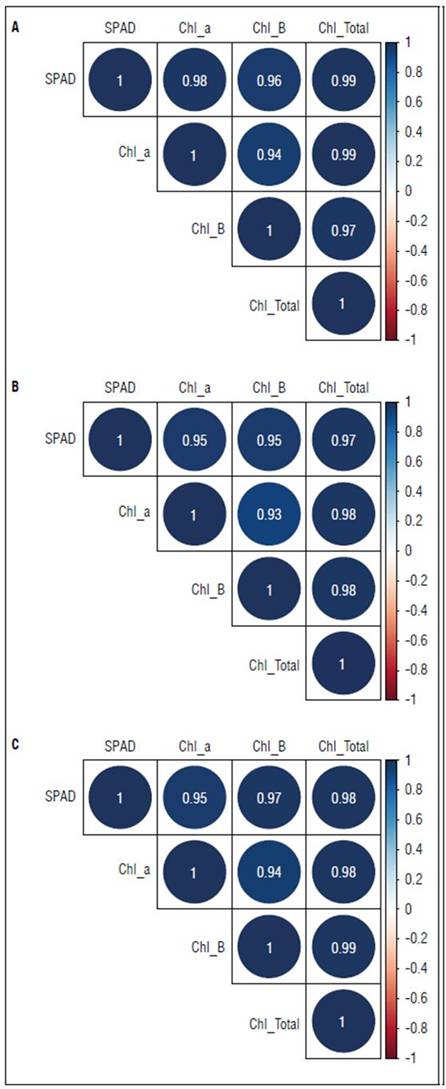
Figure 1. Pearson correlation diagram for the SPAD chlorophyll index and contents of chlorophylls-chl a, b and a+b in the blueberry cvs. Legacy (A), Victoria (B) and Biloxi (C).
The Legacy cultivar presented a total chlorophyll index that ranged between 68.2 and 73.1 SPAD units. The chl a concentration presented values that ranged between 0.77 and 1.36 mg g-1 FW, the chl b had values that ranged between 0.31 and 0.66 mg g-1 FW, and the chl a+ b) had a minimum value of 1.08 and a maximum value of 1.98 mg g-1 FW (Fig. 2).

Figure 2. Relationship between the SPAD chlorophyll index and the contents of chlorophylls-chl a (A), b (B) and a+b (C) in blueberry cv. Legacy.
The Victoria cultivar had a total chlorophyll index between 57.4 and 62.8 SPAD units. The concentration of chl a had values that ranged between 1.11 and 1.56 mg g-1 FW, chl b had values that ranged between 0.40 and 0.76 mg g-1 FW, and the chl a+ b had a minimum value of 1.57 and a maximum value of 2.32 mg g-1 FW (Fig. 3).

Figure 3. Relationship between the SPAD chlorophyll index and the contents of chlorophylls-chl a, b and a+b in blueberry cv. Victory.
The Biloxi cultivar had a total chlorophyll index that ranged between 61.2 and 68.3 SPAD units. The chl a concentration values ranged between 1.20 and 1.67 mg g-1 FW, chl b had values that ranged between 0.42 and 1.18 mg g-1 FW, and the chl a+b) had a minimum value of 1.62 and a maximum value of 2.85 mg g-1 FW (Fig. 4).

Figure 4. Relationship between the SPAD chlorophyll index and the contents of chlorophyll-hl a (A), b (B) and a+b (C) in blueberry cv. Biloxi.
The results indicated that the quantification of the chlorophyll content, either directly or indirectly, varies between cultivars of the same species. This was also observed by Deok Han et al. (2022b), who, when evaluating three blueberry cultivars, found variations in the SPAD value, depending on the cultivar, the position of the leaf, and when the measurements were taken. The same authors indicated that SPAD is a useful tool to characterize different blueberry cultivars that can determine whether they are suitable for their environment.
The leaves with light green tones were associated with low SPAD values, and dark green tones were associated with high SPAD values. Deok Han et al. (2022a) indicated that the SPAD value of leaves is related to maturity since leaves in the apical position are relatively immature, and leaves in the basal position are comparatively old, causing the total chlorophyll content to vary. Likewise, Castañeda et al. (2018) observed that, in Vitis vinifera plants, as leaves acquired darker green tones, the SPAD value increased. Therefore, the plant and the leaves must be taken into account when evaluated with these types of meters to obtain greater reliability in the results. In the present study, the leaves were taken from the middle-third, with no evidence of high variation in the data (Fig. 2, 3, 4). Similar results were described by Castillo and Ligarreto (2016), who, when evaluating the SPAD method in maize plants, concluded that the best area for taking samples is the middle-third of plants.
The results indicated that the SPAD method presented a high correlation with the content of photosynthetic pigments: chl a, b and a+b. In the evaluated cultivars, it was observed that the linear regression represented the behavior of the contents of chl a, b and a+b in terms of the total chlorophyll index (SPAD), where the R 2 value was greater than or equal to 0.90 (n=30; P≤0.001) in the three cultivars. This indicated that there was a positive relationship between the destructive method and the SPAD method (Tab. 1).
Table 1. Linear regression parameters between SPAD units and chlorophyll content in three blueberry cultivars.
| Cultivar | Variable | Intercept | P-value | slope | P-value | R 2 | Equation lineal model |
|---|---|---|---|---|---|---|---|
| Biloxi | Chl a | -3.8011 | <0.001 | 0.081 | <0.001 | 0.91 | Chl a = 0.08103*SPAD - 3.8011 |
| Chl b | -6.3354 | <0.001 | 0.1095 | <0.001 | 0.94 | Chl b = 0.1095*SPAD - 6.3354 | |
| Chl a+b | -10.116 | <0.001 | 0.1901 | <0.001 | 0.95 | Chl a+b = 0.19018*SPAD - 10.1160 | |
| Legacy | Chl a | -7.2883 | <0.001 | 0.118 | <0.001 | 0.95 | Chl a = 0.1180*SPAD - 7.2883 |
| Chl b | -3.1641 | <0.001 | 0.0515 | <0.001 | 0.91 | Chl b = 0.0515*SPAD - 3.1641 | |
| Chl a+b | -10.4368 | <0.001 | 0.1694 | <0.001 | 0.97 | Chl a+b = 0.1694*SPAD - 10.4368 | |
| Victoria | Chl a | -2.919 | <0.001 | 0.0712 | <0.001 | 0.9 | Chl a = 0.0712*SPAD - 2.9190 |
| Chl b | -2.8372 | <0.001 | 0.0569 | <0.001 | 0.9 | Chl b = 0.0569*SPAD - 2.8372 | |
| Chl a+b | -5.446 | <0.001 | 0.1229 | <0.001 | 0.94 | Chl a+b = 0.1229*SPAD - 5.4460 |
The results agreed with the behavior of other species with respect to the mathematical adjustment of their relationships, such as in soybean, where linear regression best described the relationship between SPAD readings and concentrations of photosynthetic pigments (Fritschi and Ray, 2007). However, the results differed from those reported by Castañeda et al. (2018), who indicated that, in grape leaves, polynomial equations generated the best correlation between the SPAD method and the destructive method. Donnelly et al. (2020) observed that the quadratic model showed the best fit when correlating the SPAD method and the chlorophyll content extracted from the leaves of different tree species.
This method must be validated for different species and their cultivars under field or greenhouse conditions because, as observed here, there are morphological variations, such as increased or decreased leaf thickness, physiological variations, such as a greater or lower content of photosynthetic pigments, environmental variations, such as longer or shorter exposure to light, and genotype variations (Uddling et al., 2007; Castañeda et al., 2018; Güiza-Castillo et al., 2020).
CONCLUSIONS
This study evidenced a significant correlation between the total chlorophyll index measurements (SPAD) and the concentrations of chlorophylls a, b and total in leaves from the middle-third of blueberry cultivars Legacy, Victoria and Biloxi in the vegetative stage in the high tropics. Linear regression equations allow extractable chlorophyll contents to be inferred from the total chlorophyll index (SPAD).
This research confirmed the usefulness and importance of using the SPAD method for the non-destructive determination of chlorophyll contents in different blueberry cultivars under field conditions because it is an easy, fast and efficient method.















