INTRODUCTION
Eryngium foetidum is a biennial herb belonging to the Apiaceae family, that is native to Central and Southern America and is now distributed worldwide (Ngang et al., 2014). In Colombia, is known as “culantro” or “cimarron” is used as a medicinal plant, and it has attracted the attention of researchers for its versatility as both a phytotherapeutic plant and spice herb in dishes due to its strong and fruity aromas (Leitão et al., 2020). The ease with which this herb is cultivated and propagated contributes to the high economic value of its EO in international trade markets (Paul et al., 2011). In Colombia, is found in the biogeographic regions of the Llanura del Caribe, Orinoquia, Pacífico, Valle del Cauca, and Valle del Magdalena (5-1.600 m altitude) (Bernal et al., 2015).
E. foetidum is a medicinal species whose extracts or essential oils have various ethnobotanical uses, including larvicidal (Sumitha et al., 2014), antihelmintic, anticonvulsant, antidiabetic activities (Paul et al., 2011), chemopreventive (Promtes et al., 2016; Zhang et al., 2022), antimicrobial and antioxidant (Paw et al., 2022), among others. Previous reports have demonstrated that certain EOs can produce phytotoxic activity against plants, affecting their seed germination and root and shoot growth of seedlings (Vasconcelos et al., 2022).
The trend today is to develop alternative control methods using natural products to replace non-efficient pesticides, in addition, to decrease the development of resistance to them. Due to this and the growing interest in organic farming practices, several acaricides have undergone restriction of use in the global market, such as organochlorines, organophosphates, and pyrethroids (Ellse and Wall, 2014) Consequently, the development of new agents and/or effective alternative strategies for their control is necessary. The products derived from plants are particularly attractive due to their low toxicity, scarce environmental permanence, and the complex chemistry that hinders the development of the resistances. Essential oils have also been studied as insecticides, with different biological models, such as fruit flies, weevils, among others (Ortiz de Elguea-Culebras et al., 2018).
Evaluations in vitro of acaricidal activity or resistance to synthetic pesticides have been reviewed, and they mainly focus on just one species, the one host tick, Hyalomma lusitanicum. The ixodic tick species of the genus Hyalomma belongs to the Ixodidae family and has high importance in veterinary and medicine regarding health and economy in the tropical and subtropical regions (Djebir et al., 2019; Kumar et al., 2020). Around the world, ticks and tick-borne diseases (TTBDs) are the main hurdles to enhancing livestock productivity, with global losses of approximately US$22-30 billion annually (Lew-Tabor and Rodriguez, 2016). Ticks feeding on domestic and livestock animals can result in various adverse effects, including anemia, paralysis, toxicosis, decreased quality of the leather, and transmission of many diseases of diverse etiology (Djebir et al., 2019).
Therefore, the aim of this study was to study the ixodicide capacity of Eryngium foetidum essential oil on Hyalomma lusitanicum and the phytotoxic activity of the EO and its significant components assessing their plant regulatory effect on the seedling growth of Lactuca sativa and Lolium perenne.
MATERIALS AND METHODS
Vegetal material
Eryngium foetidum plants were purchased in the Bogota market "Las Hierbas", in 2022. Taxonomic identification was performed in the University of Antioquia Herbarium (HUA) (Medellín-Colombia). The control leaves of each plant are archived as a permanent specimen in the Herbarium (No. HUA 167357).
Seeds of Lolium perenne L. and Lactuca sativa L. var. longifolia (Lam.) were provided by the Biopesticides and Natural Products Research Group, Institute of Agricultural Sciences (ICA), CSIC (Madrid-Spain).
Extraction of the essential oil (EO)
EO was obtained by the hydrodistillation method using a Clevenger type distillation equipment (Jaramillo-Colorado et al., 2012). 500 g of finely chopped leaves and stems of E. foetidum were used and immersed in boiling water by conventional heating for 4 h. The EO was separated by decanting and then anhydrous Na2SO4 (Merck) was added to remove traces of water. Finally, an aliquot of the EO (30 μL) was diluted in 1 mL dichloromethane (Panreac AppliChem) for gas chromatographic analysis (Jaramillo-Colorado et al., 2022).
Chromatography analysis
The anion-exchange chromatography was analyzed on an Agilent Technologies model 7890A GC-MS system coupled to a model 5975c mass selective detector (Palo Alto, CA) equipped with a split/split-less injection port (230°C, split ratio 20:1). Mass spectra were obtained by electron impact ionization at 70 eV energy. GC conditions were as follows: An HP-5MS capillary column (30 m × 0.25 mm id × 0.25 μm df) with 5% phenyl poly (methyl siloxane) was used. The initial oven temperature was 50°C for 2 min and then a ramp was added at a rate of 3°C min-1 up to 250°C. The carrier gas used was He, with an inlet pressure at the head of the column of 12.67 psi at a rate of 1 mL min-1, at 50°C. The mass spectra and Kovats retention indices obtained were compared with those reported in the NIST08 library database and in the literature (Adams, 2007).
Phytotoxic activity
Studies of the effects of EO and some of its major constituents on germination, radicle and cotyledons length were conducted on seeds of Lolium perenne and Lactuca sativa var. longifolia as previously described by Jaramillo-Colorado et al. (2019) with some modifications and compared with a carvone positive control (Sigma-Aldrich, 98%).
Assays were performed in 12-well plates (3.80 cm2 each). In each well, filter paper discs (Whatman® No. 1, 20 mm diameter) treated with 20 μL of EO stock solution at 0.1 mg mL-1, in ethanol, 10 seeds and 500 μL of water (0.05 mg mL-1 for the standards of the majority compounds and the control) were placed. The solvent was implemented as a blank. Subsequently, the plates were covered and placed in a plant culture chamber (26±1°C conditions with 70% RH and 16:8 h L:O photoperiods) for 7 d. A total of four replicates per treatment were carried out. Germination was observed every 24 h until the 7 d were completed. A seed was considered germinated when root protrusion was evident (Bewley et al., 2013). The germination index was calculated by comparing means with the blank. At the end of the trials, radicle (for L. sativa and L. perenne) and cotyledons (for L. perenne) lengths were measured digitally using ImageJ software (http://imagej.nih.gov/ij).
Growth and identification of H. lusitanicum
Larvae of Hyalomma lusitanicum was provided by the Department of Animal Reproduction, National Institute of Agricultural and Food Research and Technology (INIA), CSIC (Madrid-Spain) were used. These were collected from their hosts (deer) and kept in an incubator at 24±2°C and 70% RH. Taxonomic identification was performed according to Estrada-Peña et al. (2017). Larvae of 6-10 d of age were used for bioassays as described by Gonzales-Coloma et al. (2013).
Insecticidal activity
H. lusitanicum larvae were used for these assays as described by Ortiz de Elguea-Culebras et al. (2018). In Eppendorf tubes, 25 mg of cellulose (Merck Crystallised Cellulose) and 300 μL of the serial stock solutions of EO (20 to 1.25 µg µL-1), in acetone, were added and left open until the solvent evaporated in a fume hood. The contents of the Eppendorfs were added to test tubes previously containing 20 H. lusitanicum specimens and incubated at 24±2°C and 70% RH for 24 h.
After this time, mortality of H. lusitanicum larvae was counted, considering the absence of leg movement. This was corrected for target mortality according to the equation of Abbott (1925); %M=(%T-%C/100-%C)×100, where %T is the percentage of ticks killed in the treatment and %C the percentage of ticks in the negative control (target). Thymol at 10 µg µL-1 and acetone as blank were used as positive control. Each test was performed in triplicate.
Statistical analysis
In the bioassays of phytotoxic activity, it was verified whether the percentage of inhibition of germination in the seeds depended on the treatment, and the effects of E. foetidum EO on radicle and cotyledons length were studied using non-parametric analysis of variance (Mann-Whitney U test), compared with the positive control. On the other hand, using the results obtained in the acaricidal activity, the LC50 concentration was determined by ANOVA statistical analysis and a linear regression of probit analysis with a confidence level of 95%. These statistical analyses were carried out using Statgraphics Centurion® software v. 19.
RESULTS AND DISCUSSION
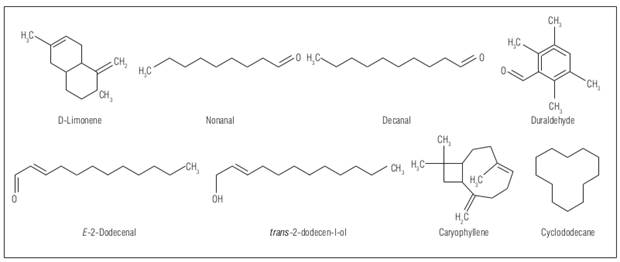
The essential oil of E. foetidum obtained by hydrodistillation presented a yield of 0.26% (w/w). Table 1 shows the main compounds found in the EO of E. foetidum. Fifteen compounds with a relative area greater than 0.5% were found, where the principal analytes were E-2-dodecenal (53.03%), trimetilbenzaldehyde (duraldehyde) (14.8%), cyclododecane (4.4%), trans-tetradec-enal (3.9%), decanal (3.6%), trans-2-dodecen-1-ol (3.0%) and D-limonene (1.5%). Some structure can be seen in the figure 1.
Table 1. Major compounds found in the essential oil of E. foetidum, obtained by GC-MS.
| Peak, No. | Compound | tR (Min) | Ik (HP-5) | Relative area (%) |
|---|---|---|---|---|
| 1 | a-Pinene | 7.15 | 937 | 0.3 |
| 2 | b-Ocymene | 10.71 | 1022 | 0.3 |
| 3 | D-Limonene | 10.87 | 1026 | 1.5 |
| 4 | g-terpinene | 12.19 | 1062 | 0.2 |
| 5 | Nonanal | 13.76 | 1099 | 1.2 |
| 6 | Undecane | 14.12 | 1100 | 0.5 |
| 7 | Decanal | 18.99 | 1206 | 3.6 |
| 8 | Trimetilbenzaldehyde (duraldehyde) | 25.43 | 1313 | 14.8 |
| 9 | E-2-Dodecenal | 27.46 | 1408 | 53.0 |
| 10 | Caryophyllene | 28.04 | 1417 | 0.6 |
| 11 | trans-2-dodecen-1-ol | 30.51 | 1469 | 3.0 |
| 12 | Cyclododecane | 30.72 | 1502 | 4.4 |
| 13 | Dodecanoic acid | 34.61 | 1565 | 1.5 |
| 14 | Caryophyllene oxid | 35.96 | 1582 | 1.5 |
| 15 | trans-Tetradec-enal | 38.17 | 1673 | 3.9 |
Ik: Kováts index performed in apolar column HP-5 (5% phenyl -95% polymethyl siloxane) (30 m × 0.25 mm di × 0.25 um df).
Table 2 shows the phytotoxic effects of E. foetidum essential oil, and commercial standards of some compounds present in the EO, on seeds of L. perenne and L. sativa. These show that germination was inhibited between 3.3 to 25.2% on L. sativa and 3.4 to 25.0% on L. perenne after 7 d. The results indicate that neither the EO nor the compounds acting individually have phytotoxic activity as they do not exceed 50.0% inhibition of germination. There was less variability and greater consistence in radicle elongation (8.4-5.6%) in L. sativa compared to L. perenne (42.4-0.9%) and similar results in cotyledons (35.0-2.4%), showing greater susceptibility of L. perenne to phytotoxic activity of E. foetidum EO and its compounds.
Table 2. Inhibitory activity (%) of the essential oil of E. foetidum and some of its major compounds on the germination of L. perenne and L. sativa seeds.
| Compound | Conc. | L. sativa | L. perenne | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Germinationa | Growtha | Germinationa | Growtha | |||||||
| 72 h | 120 h | 168 h | Radicle | 72 h | 120 h | 168 h | Radicle | Cotyledons | ||
| E. foetidum | 0.10 | 46.7±16.8b | 78.2±6.2b | 80.6±2.2b | 91.4±6.3 | 64.7±16.7b | 66.7±6.4b | 75.0±6.1b | 79.2±7.1b | 76.9±7.6b |
| β-pinenec | 0.05 | 58.4±11.0 b | 71.8±7.6b | 81.2±3.4 | 93.5±7.2 | 67.7±17.3b | 76.8±9.1b | 86.2±2.5 | 85.4±6.7 | 87.6±5.4 |
| D-limonenec | 0.05 | 60.8±12.5b | 79.4±9.4b | 90.2±6.1 | 92.7±8.3 | 160.0±35.9 | 92.6±7.3 | 96.6±4.8 | 86.9±6.8 | 97.6±6.0 |
| p-cymenec | 0.05 | 63.9±16.3 b | 81.3±11.3 | 89.9±8.3 | 93.6±7.4 | 90.0±26.3 | 70.4±8.7b | 79.3±2.1b | 82.4±7.7 | 82.5±5.4 |
| Duraldehydec | 0.05 | 41.7±12.4 b | 78.2±6.8b | 74.8±3.2b | 91.8±6.7 | 40.0±19.4b | 62.7±8.4b | 75.9±3.1b | 57.6±4.9b | 65.0±14.6b |
| 2-Dodecenalc | 0.05 | 38.4±10.7 b | 76.4±7.1b | 86.2±4.5 | 92.0±5.1 | 100.0±28.3 | 77.8±8.9b | 89.7±2.8 | 92.6±7.6 | 95.8±6.4 |
| Caryophyllenec | 0.05 | 100.0±24.5 | 92.0±14.8 | 96.7±8.5 | 94.4±9.7 | 180.0±37.8 | 92.6±7.5 | 93.1±3.5 | 99.1±7.3 | 109.2±6.3 |
| Positive control (carvona) | 0.05 | 29.7±12.3b | 78.1±6.1b | 78.5±3.7b | 91.6±3.9 | 58.0±14.5b | 63.5±6.2b | 67.2±7.3b | 75.2±7.4b | 51.4±6.2b |
Conc., concentration (mg mL-1). a percentage of germination and growth with respect to blank (solvent) b significance with respect to the blank according to the Mann-Whitney U-test (P<0.05). c Majority compounds.
Figure 2 presents the acaricidal activity of E. foetidum essential oil and the standards of its major compounds on ticks of the species Hyalomma lusitanicum (% mortality). The essential oil of E. foetidum showed 100% mortality at concentrations of 20 to 10 µg µL-1. At the same time, individual compounds showed 100% mortality at concentrations of 10 to 5 µg µL-1. The duraldehyde compound was the only one showing 32.8%±2.1 mortality at 10 µg µL-1. Positive control mortality ranged from 92.0 to 10 % at concentrations of 10 to 0.6 µg µL-1.

Figure 2. Curve of insecticidal activity of E. foetidum EO, and its major compounds on H. lusitanicum larvae.
Table 3 presents the LC50 (µg µL-1) by Probit analysis (P>0.05) of the E. foetidum EO and the individual compounds tested. These showed insecticidal activity as they exhibited toxicity at low concentrations (between 1.9 and 8.1 µg µL-1). It should be noted that neither duraldehyde nor the positive control had an LC50 calculated, as there were insufficient results to determine their value statistically.
Table 3. Results obtained from the determination of the LC50 for Eryngium foetidum EO, and its major compounds, through a Probit analysis.
| Compounds | LC50 | (FL, 95%) | |
|---|---|---|---|
| Lower limit | Upper limit | ||
| E. foetidum | 4.2 | 3.8 | 5.3 |
| β-Pinene | 3.4 | 2.9 | 4.0 |
| D-Limonene | 4.3 | 3.9 | 5.4 |
| p-Cymene | 7.2 | 6.4 | 8.1 |
| Duraldehyde | - | - | - |
| 2-Dodecenal | 2.4 | 1.9 | 2.6 |
| Caryophyllene | 3.2 | 2.2 | 4.4 |
FL: Fiducial limits, n=5.
The main compounds found in the Eryngium foetidum EO were E-2-dodecenal and trimetilbenzaldehyde (duraldehyde), an aldehyde and benzenic compound, respectively. These substances are effective to treat diseases and can prevent oxidative deterioration in food (Rodrigues et al., 2022).
The chemical composition in this study was similar to the results obtained through other essential oils of leaves from E. foetidum from other countries, i.e., in the EO from Nigeria and Brazil, where the principal compounds found were E-2-docecenal and tetradecenal (Thomas et al., 2017; Rodrigues et al., 2021). In contrast, a study in India reported trimetilbenzaldehyde as the main component (Chandrika et al., 2015).
To date, there is no previous research on the phytotoxic activity of E. foetidum EO. In this study, EO significantly inhibited radicle growth of L. sativa and L. perenne and cotyledons growth of L. perenne when compared to the blank sample. This could be due to the chemical composition of EO. In this study, the phytotoxic potential of the major compounds duraldehyde, and 2-dodecenal were also evaluated for the first time. The latter was not phytotoxic against seeds of L. sativa and L. perenne, because it showed a similar behavior to the target according to the comparison of variances by the non-parametric test performed; in contrast to duraldehyde which considerably inhibited germination in the seeds studied.
Plant metabolites with phytotoxic effects are capable of inhibiting seed germination, this effect is associated with several mechanisms, including inhibition of DNA synthesis and cell proliferation, inhibition of enzymes, photosynthesis and seedling growth, alteration of membrane, permeability, and respiration (Rys et al., 2022).
Oxygenated terpenes exhibit a primary role in the phytotoxicity of an EO, p-cymene, β-pinene, are the most effective monoterpenes, with significant phytotoxicity evident in the EOs of many plants (Abd-ElGawad et al., 2020). Chowhan et al. (2013) showed that β-pinene inhibited germination, leaf and root length in herbaceous weeds, results that coincide with those obtained in this research.
The essential oil of E. foetidum, b-pinene, D-limonene, p-cymene, duraldehyde, and caryophyllene were very active against the larvae of H. lusitanicum with 100% mortality at concentrations between 2.5-10.0 µg µL-1. At the time of this investigation, no reports of the essential oil from E. foetidum as an acaricide on H. lusitanicum were found. Ortiz de Elguea-Culebras et al. (2018) reported strong insecticidal activity of essential oil from lavandin (Lavandula × intermedia or L. × hybrida var. Super) and cotton lavender (Santolina chamaecyparissus L.) against H. lusitanicum.
Monoterpenes such as carvacrol, thymol, limonene, limonene oxide, and pulegone exhibited larvicidal and toxic effects to engorged females of R. (B.) microplus (De Oliveira-Cruz et al., 2013).
In this research, it is worth highlighting the importance and potential of the compounds found in the essential oil of E. foetidum due to its composition of aldehydic and benzene compounds, rare molecules in essential oils. Forbes et al. (2014) evaluated eryngial (trans-2-dodecenal), a bioactive compound from E. foetidum, as an anthelmintic using infective third-stage larvae of Strongyloides stercorali. There was a significant difference between the 24 h LC50 values of trans-2-dodecenal (0.461) and ivermectin (2.251) in vitro.Erdem et al. (2015) reported the eryngial as one the most important and major compounds of genus Eryngium plant essential oil, it possesses a significant antibacterial effect, and the 2,3,6-trimethylbenzaldehyde (duraldehyde) showed antibacterial activity (Demirci and Özkan, 2014).
Besides, Sumitha et al. (2014) showed the mosquito larvicidal efficacies of the essential oil from E. foetidum against the fourth instar larvae of Aedes albopictus Skuse (Diptera: Culicidae). The essential oil showed an excellent larvicidal effect, and the LC50 value in 24 h was 33.3 ppm (LC90 = 57.7 ppm).
CONCLUSIONS
The results obtained in this study showed that the essential oil of E. foetidum has a great biocidal potential to develop natural products to control ticks of the species Hyalomma lusitanicum, due to its volatile chemical composition rich in terpenes and aldehyde and benzene compounds. in addition, the EO does not inhibits radicle growth of L. sativa and L. perenne and cotyledons growth of L. perenne.