INTRODUCTION
Yam (Dioscorea) is a crop whose production continues to be led by the African continent. According to the Food and Agriculture Organization of the United Nations (FAO, 2021), Africa represented 97.8% of global yam production, while, for the continents of the Americas, Oceania and Asia, their contributions were 1.0, 0.6 and 0.2%, respectively. In Colombia, the largest production is concentrated in the Caribbean region, especially in the departments of Sucre, Bolivar and Cordoba, the latter department being the one that recorded the highest production of yams in 2022 with 123,667.3 t, represented in the varieties of diamond or creole yam (Dioscorea alata[L.]) and hawthorn yam (Dioscorea rotundata[Poir.]) (MinAgricultura, 2022). Despite the potential of yam cultivation, there are several challenges when establishing new crops. One of these difficulties is related to the natural dormancy of the tuber, which is reflected in the absence of sprouting and can last several months in the case of hawthorn yam (D. rotundata) (Wickham, 2019). Therefore, it must be stored or planted while it is still in a period of rest or dormancy (Sánchez-López et al., 2021; Datir et al., 2024). This leads to the yam suffering from fungal attacks if there is not adequate storage and in the case of plantation, seed tubers losses are generated in the field, causing a decrease in sowing density and uneven crops, which sometimes the final result is economic losses for producers due to the management difficulties that this situation entails.
Tuber dormancy is an inherent process in plants, governed by a precise balance of chemicals that allows them to withstand challenging environments and restart their development when circumstances permit. Abscisic acid is the primary regulator of this state of rest, curbing cellular activity and preserving tubers in a phase of inactivity (Davies, 2010; Guo et al., 2021). With the improvement of environmental conditions and the reduction of abscisic acid levels, gibberellins take on the leading role, driving cellular division and growth that results in the emergence of sprouts. This control mechanism ensures that tubers only emerge when conditions are optimal for the success of the new plant (Kumari et al., 2024). For this reason, different methods of breaking dormancy in yam tubers have been studied, including the use of external factors such as temperature (Sanada et al., 2018;Nwogha et al., 2022). In turn, the use of synthetic plant growth regulators (PGRs), which are analogs of phytohormones and have functions in the regulation of various biochemical processes at the cellular level in plant organisms, has been investigated (Alcántara et al., 2019). Among the reported PGRs is thiourea at 1, 2, and 6%, which has been used in tuber species to stimulate bud sprouting (Hamadina and Craufurd, 2015; Zhu et al., 2023). Another reported PGR is mepiquat chloride widely used in cotton (Chen et al., 2018), which induces sprouting in yam tubers with a maturity level of 326 d after planting with a dose of 1.0 g L-1 with greater effectiveness (Sánchez-López et al., 2021).
To date, there are few studies on the application of different PGRs on yam tubers that act as dormancy inhibitors and thus offer a solution to producers who suffer seed losses before and during plantation. Due to the above, it is necessary to continue researching and developing a technology that reduces the dormancy period and ensures that field planting is carried out with sprouted sections of the tuber, since this will reduce losses and minimize the deterioration of the quality of the tuber. This and uniformity of the plantations is achieved to achieve better crop management and increase the percentage of export-type quality tubers. Therefore, the objective of this research was to evaluate plant growth regulators (PGRs) in tuber sections of commercial hawthorn yam to induce sprouting.
MATERIALS AND METHODS
The experiment was carried out in the Laboratory Unit of the Turipaná-AGROSAVIA Research Center, Cerete-Colombia, 14 m a.s.l., located at 8°31'16'' N and 5°58'11'' W.
Two PGRs reported in the literature as dormancy inhibitors were evaluated and two concentrations were used to define the six treatments, including two absolute controls (Tab. 1).
TABLE 1. Treatments to evaluate the effect of regulators of plant growth in the breaking of dormancy yam tubers.
| PGRs | Dose | Abbreviation |
|---|---|---|
| Thiourea | 1.0 g L-1 | 1thiourea |
| Thiourea | 2.0 g L- | 2thiourea |
| Mepiquat chloride | 1.0 mL L-1 | 1MC |
| Mepiquat chloride | 2.0 mL L-1 | 2MC |
| Control with humidity | distilled water | Control+humidity |
| Control without humidity | --- | Control-humidity |
The collected yam tubers were subjected to preparation that consisted of removing the rootlets present on the surface and subsequent washing with drinking water. Next, they were divided into their apical, middle and basal sections. Each section was subdivided into 4 to 7 small-sized of 50 g for disinfection with 2% sodium hypochlorite. To do this, the small-sized were immersed for 1 min in the solution and subsequently rinsed to remove any traces of sodium hypochlorite.
The PGR solutions were prepared at concentration 1.0 and 2.0 g L-1 thiourea, and 1.0 and 2.0 mL L-1 of mepiquat chloride. The small-sized of each section was immersed for 60 min in the different PGRs treatments. Once the PGR was applied, sanitary protection was carried out with a fungicide composed of 4.0 g of metalaxyl-M and 64 g mancozeb (4.0 g L-1), depositing the small-sized for 5 min. Then, they were spread on a mesh bag at room temperature for 8 to 12 h in order to promote drying.Finally, five small-sized individuals were placed in plastic boxes measuring 21×16×10 cm, containing moistened sawdust, and stored on shelves in a bioassay room without artificial light at a temperature of 28±2°C, with a relative humidity of 80%. Three replicates were conducted for each PGR treatment and section.
Observations were made at 7, 14, 21, 29, 36 and 43 d after storage. To verify the sprouting of the sections, the boxes were uncovered, and signs of sprouting or bud formation were identified visually on the surface of the sections.
Statistical analysis
A completely randomized design was used, with three sections (apical, middle, and basal), six treatments (hormones), six observation times (7, 14, 21, 36, and 43 days of storage), and three replications. Each experimental unit consisted of five small-sized individuals, which were subjected to analysis of variance to determine the treatment response. In cases where differences were detected at a significance level of 0.05, mean separation tests were performed using Tukey's HSD test. Data analysis was conducted using the statistical package SAS v.9.4.
RESULTS AND DISCUSSION
The analysis of variance indicated significant differences between sections of the commercial hawthorn yam evaluated. In figure 1, is observed the number of buds sprouted by each section.
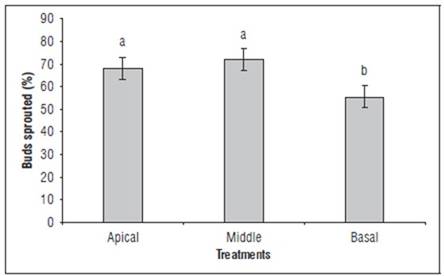
FIGURE 1. Buds sprouted of the seed tuber yam for section. Average of PGR treatments, days of observation, replicates and small-sized tuber per experimental unit. Different letters indicate significant differences according to the Tukey's HSD test (P<0.05). Vertical bars in columns indicate the standard error (n=540).
This suggests that, although the applied treatments or inherent tuber conditions may promote relatively uniform sprouting in the apical and middle sections, there are factors that differentially influence the basal section. The distribution of nutrient reserves, tissue maturity, presence of sprouting inhibitors, or phytohormonal activity may play a role in this variability (Shangguan et al., 2020; Guo et al., 2021; Nwogha et al., 2023).
Additionally, the significant difference in sprouting between the middle and basal sections of the tuber may be related to the utilization of carbohydrate reserves for respiration and transpiration, leading to cell structure breakdown in the basal section. This could explain why the basal section exhibits lower sprouting capacity compared to the upper sections, as suggested by Santos-Cáceres et al. (2021) who provide evidence of how carbohydrate reserve consumption during storage can affect cellular structure and ultimately tuber sprouting capacity.
From a practical perspective, these findings underscore the need to consider specific strategies to enhance sprouting in the basal section. This may include adjustments in growth regulators treatments, storage practices, and planting techniques, aimed at optimizing the production and management of this important crop.
In figure 2 indicated significant differences between PGRs treatments. The 1Thiourea was the most effective treatment with a sprouting response of 91%, followed by 2Thiourea with 86% sprouting. On the contrary, there was a significant difference between treatments 1MC and 2MC with mepiquat chloride for all sections and all observations, achieving sprouting rates of 74 and 83%, respectively.
All treatments, except 1MC, showed a significant difference compared to control+humidity, which corresponded the absolute control with water. This demonstrates a clear effect on sprouting speed when applying different doses of PGRs compared to a substrate containing only water. However, moisture alone favors conditions for sprouting to occur, albeit to a lesser extent as observed in this study. In many cases, adequate moisture is essential for PGRs to be effective (Taizet al., 2023). Moisture helps maintain cellular turgidity, which is necessary for tissue growth and expansion (Lim et al., 2020). Additionally, moisture can facilitate the absorption and distribution of PGRs within the plant, potentially enhancing their effect Bhatla and Lal (2023). On the other hand, excess moisture or stagnant water conditions can reduce the effectiveness of PGRs and may create a favorable environment for disease development, which could counteract the benefits of PGRs.
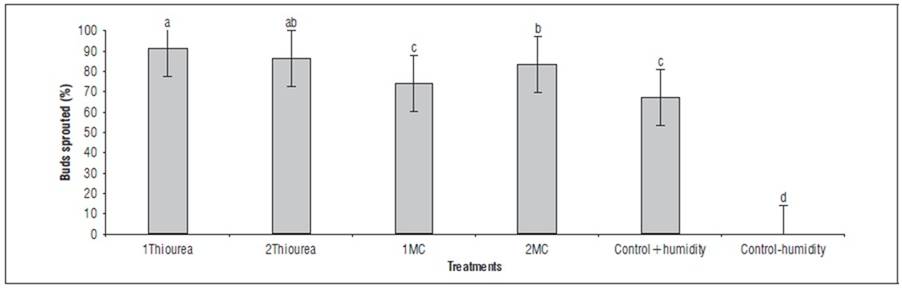
FIGURE 2. Buds sprouted of yam seed tubers treated with PGRs. 1Thiourea, 1.0 g L-1 of thiourea; 2Thiourea, 2.0 g L-1 of thiourea; 1MC, 1.0 mL L-1 of mepiquat chloride; 2MC, 2.0 mL L-1 of mepiquat chloride; control+humidity, control with humidity; control-humidity, control without humidity. Average of tuber sections, days of observation, replicates and small-sized tuber per experimental unit. Different letters indicate significant differences according to the Tukey's HSD test (P<0.05). Vertical bars in columns indicate the standard error (n=270).
The results of the PGR indicated that thiourea (1Thiourea and 2Thiourea) has a positive stimulus in the sprouting of the tuber in the apical and middle section, where there is no significant difference for the dose. However, it does not have the same effect in the basal section with less sprouting, where there is a significant difference with respect to the apical and middle sections. In the case of mepiquat chloride (1MC and 2MC), these presented a positive effect on tuber sprouting in the apical and middle part, where there was no significant difference for both treatments; but, unlike thiourea, mepiquat chloride manages to stimulate sprouting in the basal (2.0 mL L-1) (Fig. 3).
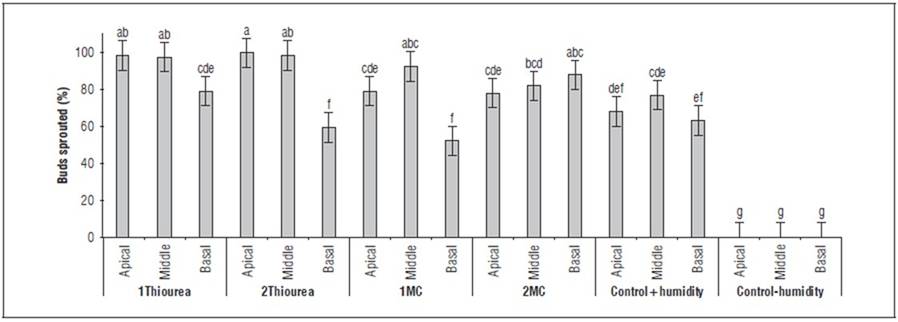
FIGURE 3. Buds sprouted (%) of yam seed tubers treated with PGRs and sections. 1Thiourea, 1.0 g L-1 of thiourea; 2thiourea, 2.0 g L-1 of thiourea; 1MC, 1.0 mL L-1 of mepiquat chloride; 2MC, 2.0 mL L-1 of mepiquat chloride; control+humidity, control with humidity; control-humidity, control without humidity. Average of days of observation, replicates and small-sized tuber per experimental unit. Different letters indicate significant differences according to the Tukey's HSD test (P<0.05). Vertical bars in columns indicate the standard error (n=90).
Reports by Epping and Laibach (2020) confirm that sprouting involves the activity of a layer of meristematic cells located just under the skin of the tuber, giving rise to a set of shoots, which generally occurs in the apical section of these tubers. Yams exhibit proximal dominance. When the first shoots form, the formation of subsequent shoots is suppressed. Therefore, the apical part of the tubers is preferred as seeds because they sprout earlier than the middle and basal regions. This is why techniques involving tuber sections have been developed to overcome low multiplication rates (Velázquez-Hernández et al., 2022). In this research, it was observed that the apical and middle sections in treatments 1Thiourea and 2Thiourea are similar; however, less sprouting was observed in the basal part, which can be detrimental for extensive areas when considering significant losses in this section of the tuber. Therefore, the analysis and response of treatment 2MC provide an alternative with a more uniform sprouting response, allowing for the handling of large seed volumes without generating losses or irregularities in the field. In practice, a producer must separate the seed sections to carry out differential treatments, using one molecule for the apical and middle sections and another molecule for the basal part. Although this practice may seem confusing and challenging to apply, it presents an alternative with close to 100% efficiency in sprouting yam tuber seeds.
In table 2, the results of the percentage of sprouting in tuber sections treated with different PGRs over a 43-day observation period are presented. Treatment 1thiourea showed the highest percentage of sprouting on all observation days, reaching 98% by day 36. In contrast, treatment control-humidity did not show any sprouting on any of the observation days. Treatments 2Thiourea, 1MC, 2MC, and control+humidity exhibited varying effectiveness in promoting sprouting, with 2thiourea and 2MC being more effective than 1MC and control+humidity. The effectiveness of treatments varied over time, with some showing a gradual increase in the percentage of sprouts. These results suggest that the PGRs used in treatments 1Thiourea, 2Thiourea, and 2MC are the most effective in promoting tuber sprouting under the conditions of this experiment.
TABLE 2. Buds sprouted by treatment on each of the observation days for the tuber sections.
| Treatment PGR | Observation days | Sprouted buds (%) |
|---|---|---|
| Thiourea, 1.0 g L-1 | 7 | 67 cdef |
| 14 | 93 ab | |
| 21 | 96 a | |
| 29 | 96 a | |
| 36 | 98 a | |
| 43 | 98 a | |
| Thiourea, 2.0 g L-1 | 7 | 64 def |
| 14 | 84 abcde | |
| 21 | 89 abcd | |
| 29 | 89 abcd | |
| 36 | 93 ab | |
| 43 | 93 ab | |
| Mepiquat chloride, 1.0 mL L-1 | 7 | 56 fg |
| 14 | 60 efg | |
| 21 | 73 abcdef | |
| 29 | 82 abcde | |
| 36 | 84 abcde | |
| 43 | 91 abc | |
| Mepiquat chloride, 2.0 mL L-1 | 7 | 56 fg |
| 14 | 76 abcdef | |
| 21 | 84 abcde | |
| 29 | 89 abcd | |
| 36 | 96 a | |
| 43 | 96 a | |
| Control with humidity | 7 | 38 g |
| 14 | 51 fg | |
| 21 | 69 bcdef | |
| 29 | 82 abcde | |
| 36 | 87 abcd | |
| 43 | 89 abcd | |
| Control without humidity | 7 | 0 h |
| 14 | 0 h | |
| 21 | 0 h | |
| 29 | 0 h | |
| 36 | 0 h | |
| 43 | 0 h |
Average of tuber sections, replicates and small-sized tuber per experimental unit. Different letters indicate significant differences according to the Tukey's HSD test (P<0.05).
Thiourea is a chemical compound that has been used in agriculture as an agent to break dormancy in tubers such as Solanum tuberosum (Ranabhat et al., 2021; Gul and Iqbal, 2023). This molecule can act as a reducing agent and has been reported to influence the activity of enzymes involved in plant metabolism, which can affect tuber dormancy (Mani et al., 2013). Additionally, it has been suggested that thiourea can inhibit catalase activity, which in turn could influence hydrogen peroxide (H2O2) concentration in plant cells (Hendricks and Taylorson, 1975). Hydrogen peroxide is a molecule that may be involved in dormancy signaling and plant growth regulation (El-Maarouf-Bouteau and Bailly, 2008). According to the results, treatments 1Thiourea and 2Thiourea showed significant sprouting compared to control+humidity and control-humidity.
On the other hand, mepiquat chloride partially inhibits an enzyme involved in gibberellic acid (GA) biosynthesis, thereby reducing dormancy compared to control+humidity, which corresponds to the absolute control with moisture. This is confirmed by the results where 2MC showed 76% sprouting at 14 d, compared to 50% for the absolute control with moisture. Reports by Rao and George (1990) mention that gibberellic acid at 1,000 mg L-1 for 2 h extends dormancy in yam by 4 months.
It is important to consider this type of strategies in scenarios of climate variability, where the opportunity for water resources may be limited and greater use is required during the first phases of crop establishment.
By achieving uniform sprouting, management alternatives can be provided, especially in the application of irrigation, synchronization of nutrition at the time of sowing, avoiding losses of fertilizers, efficient management of weeds in the crop and early detection of pathogens for a good handling and control, achieving at the end of the production cycle, uniform harvests, minimal losses, good quality products and guarantee for the market and marketers.
CONCLUSION
The treatments evaluated showed a significant difference in the percentage of sprouting and reduction in dormancy time, compared to the absolute control. Therefore, a positive effect on the breaking of dormancy was demonstrated by the application of PGRs for the commercial hawthorn yam variety (D. rotundata). The best treatment for the apical and middle section was with thiourea 2.0 g L-1 reaching 100% and more than 90% sprouting respectively, and for the basal section with mepiquat chloride 2.0 mL L-1 with 88% sprouting.















