INTRODUCTION
The lemon verbena (Aloysia citriodora (Palau) or Aloysia triphylla [L'Hér.] B.) is native to South America, North Africa and Southern Europe (Bahramsoltani et al., 2018). It belongs to the Verbenaceae family, and possesses antimicrobial (Oliva et al., 2010), and antiviral properties (Meneses et al., 2009); it has been used in infusions for control fever, insomnia, anxiety, some heart problems, and gastrointestinal disorders (Argyropoulou et al., 2007). This utilization dates back to the 17th century, giving the species ethnopharmacological importance, in addition to other uses in the cosmetic and food industries (Bahramsoltani et al., 2018).
The major components of the plant are geranial (27.3 %), neral (22.5 %), geraniol (6.2 %), bicyclogermacrene (5.2 %) and nerol (4.9 %) (Rojas, et al., 2010). According to Stashenko et al. (2003) abundant citral, nerol, and geraniol, have been found in cultivated plants in Colombia; in addition, phenolic compounds, and flavonoids such as chrysoeriol, luteolin, and phenylpropanoid derivatives have been identified as active components (Leyva-Jiménez et al., 2018). These compounds have been successfully used in the in vitro treatment of bacteria (Rojas et al., 2010), viruses (Aurori et al., 2016), as antioxidants (Leyva-Jiménez et al., 2020), in diabetes treatments (Diez-Echave et al., 2020), in tumor prevention (Salama et al., 2021), and in fruit postharvest (Fontana et al., 2021; Shirzad et al., 2021).
Although the plant has been extensively studied for therapeutic and medicinal uses, as well as the composition of essential oils, there is no research related to its phenological stages nor the insects associated with each stage for Colombia. The aim of the study is to improve this gap of knowledge and to generate better agronomic practices for its cultivation in the country.
MATERIALS AND METHODS
The study was conducted at El Jazmin farm, municipality of Santa Rosa de Cabal, Colombia, located at 1,650 m a.s.l, with a relative humidity of 78%, temperature of 20ºC, annual rainfall of 2,620 mm, daily sunshine of 3.6 h, and wind speed of 4 km h-1 with predominant west direction (data from the Veracruz station, IDEAM, 2023).
Cuts of 15 cm long obtained from adult plants were rooted during 20 d before being transplanted to the field. The experimental unit was subdivided in 18 subplots of 15 plants each; the distance between plants was 0.5 m, and 1.0 m between rows. The terrain slope was of 30%, having soils derived from volcanic ash with an organic matter content upper than 8%. Crop management was based on agroecological practices, being the fertilization with organic matter and the weed control using manual tools.
The modified BBCH scale (Arcila et al., 2001) was used to describe the phenological stages, establishing a uniform coding to describe growth stages only six stages were taken because the species does not fruit under local environmental conditions, and an additional stage corresponding to floral senescence was included. In the nursery and field phase, 10% of the plants were evaluated weekly by measuring the branches and it was established as a parameter when 50% of them showed the morphology described in the scale, including the reproductive stage where flowers were open.
For the evaluation of the arthropofauna, the methodology proposed by Wolf-Echeverry (2006) was used, monitoring weekly all the plants and during the identified phenological stages, including swabbing at 20 cm from the soil surface. For small insects, pollinators, parasitoids and ants, suction bottles were used. Identification was made to the levels of order, family, and in some cases even genus, using taxonomic keys (Triplehorn and Johnson, 2005) and with the help of specialists from the areas of Biology and Agronomy of the Universidad del Quindío and the Universidad de Caldas (Colombia). Finally, all specimens collected were deposited in the Entomological Collection of the Corporacion Universitaria Santa Rosa de Cabal called CUS-E (Insects) with the Registro Unico Nacional de Colecciones Biologicas (RNC) number 279 (December 15, 2021).
RESULTS AND DISCUSSION
Determination of phenological stages
During 32 weeks of evaluation, seven growth stages were identified, five corresponding to the vegetative phase (Vo to V4), and two to the reproductive one (R1 and R2, Tab. 1). The V0 or nursery stage began with slow growth, developing shoots from the third week after sowing, the phase culminated with seedlings with an average of five roots, and a length of 1.22 cm (Fig. 1), under these conditions the emission of shoots was between 2 and 3 weeks, and the emission of roots was between 6 and 8 weeks, for 95 and 73% of the seedlings evaluated, respectively.
Table 1. BBCH scale for the description of A. tryphilla phenological stages.
| Main growth stage 0 (V0) | Vegetative propagation |
|---|---|
| 00 | Orthotropic or plagiotropic cuttings, with more than three knots, 15 cm long, without leaves |
| 01 | Seeded cutting, without sprouts or callus formation |
| 03 | Callus formation on cuttings. Rounded green buds. Visible on stems |
| 05 | Bud formation on the cutting |
| 06 | Root formation in the cutting |
| 07 | Formation of buds on cuttings, with 2 or 3 nodes and branched roots |
| 09 | Cuttings with roots 6 to 7 cm long and shoots with 4 to 5 nodes |
| Main growth stage 1 (V1) | Development of primary branches |
| Main growth stage 2 (V2) | Development of secondary branches, plants in the field |
| Main growth stage 3 (V3) | Elongation of primary and secondary branches, initiation of tertiary branch development |
| Main growth stage 4 (V4) | Elongation of primary, secondary and tertiary branches, and development of quaternary branches |
| Main growth stage 5 (R1) | Development of floral primordia and flower anthesis |
| Main growth stage 6 (R2) | Flowering and flower senescence |
The percentage of rooting in contrast to that obtained in shoots is explained by the difficulty of the species to form roots (Potocnjak, 2003), generating vegetative buds even in plants without root development, behavior caused by the reserves accumulated in the cutting, which cannot sustain the seedling in formation, subsequently causing the death of the shoots (Gaibor-Tulcanaz, 2016). This condition specifically for Colombia generates a difficulty in the propagation of the species because the required environmental conditions are not present for the plant to go through the stages of seed production and natural defoliation, determining characteristics not only for growth, but also for the synthesis of secondary metabolites (Aliniaeifard et al., 2010; El-Hawary et al., 2012).
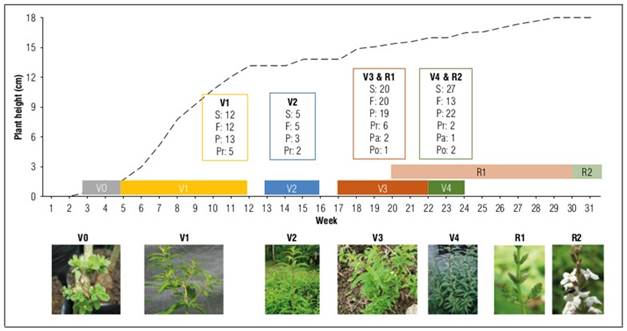
Figure 1. Relationship of plant phenology, insects, and feeding habits. S: species, F: families, P: phytophagous, Pr: predators, Pa: parasitoid, Po: pollinators.
Stage V1 occurred from week 5 to week 12 and was characterized by an increase in the number and length of primary branches, culminating with seedlings that presented an average of 5 primary branches and an average length of 13.2 cm. Stage V2 started from week 13 to week 16, during which time a constant number of 5 primary branches was maintained and the appearance of one secondary branch per week, this stage ended with 7 secondary branches, which on average had a length of 2.5 cm. Stage V3 began in week 17 and lasted until week 22, and at the end of this stage, plants with 11 secondary branches of 5.5 cm and 3 tertiary branches of 1.2 cm in length were observed. Stage V4 lasted from week 22 to week 24, showing a constant increase in growth and the number of secondary and tertiary branches, accompanied by the elongation of buds in quaternary branches, which on average had 6.8 branches per plant and a length of 1.2 cm. (Fig. 1).
Stage R1 corresponded to pre-flowering, which started with the appearance of floral primordia until anthesis; this stage occurred from the 20th week of evaluation and overlapped with stage V3 (Fig. 1). The R2 stage corresponded to flowering, from anthesis to senescence. It took place between weeks 30 and 32 after planting, and the period between flowering and floral senescence was 7 d (Fig. 1).
Although a correlation was not established between climate variables and the vegetative development of the species, the constant growth during the evaluation period characterized by the increase in the number and length of the branches could be directly conditioned by abiotic factors such as temperature, which can structurally and anatomically modify plants, favoring the rate of appearance of nodes and leaves when the ranges are optimal for the cultivated species (Pant et al., 2021).
Likewise, the temperature in the cultivation area has a direct influence on the essential oil content in different aromatic plants; specifically in A. citriodora the content of chlorophyll a, chlorophyll b, carotenoids and flavonoids increase between 5 and 10ºC; similarly, at 25ºC oxygenated monoterpenes predominate (Rafiee et al., 2019). This aspect in the study area, where the average temperature is 20ºC, would be conditioning the amount of metabolites, anthocyanins, lycopene, soluble proteins and polyphenols, but would allow a better use of fresh foliage, without affecting the growth and productivity of plants due to high temperatures or water stress. However, this topic requires a deeper analysis since the development of plants as well as the biosynthesis and storage of secondary metabolites are the result of adaptive processes and survival that involve the interaction between genetics and environmental factors (Pérez-Ochoa et al., 2023).
Also the edaphoclimatic conditions of the area where the species is grown and the type of fertilization have significant effects on the phenology of the plant, even generating delays in the beginning of the reproductive stage, as reported by Guzmán-Rivera et al. (2004) for the species Lippia alba cultivated in Colombia, where it was observed that the plant started the reproductive phase before 50 days after transplanting (DAT), with two peaks defined at 72 and 133 d in the municipality of Candelaria, Valle del Cauca, while in Pereira, Risaralda it occurred at 150 DAT. However, in the aforementioned areas, the R2 stage occurred simultaneously with the vegetative stage, which lasted until flower senescence, as it happened with A. citriodora.
The variation in time between the vegetative and reproductive stages it is important to define the harvest time, because it is directly linked to the amount of essential oil produced and its chemical composition, a determining aspect for the target market. According to Shahhoseini et al. (2013), flowering plants have the highest essential oil content, meanwhile the harvest of material in the vegetative stage is important for obtaining herbal products, which is how the plant is marketed mainly in Colombia; however, it should be considered that the dry weight of leaves and stems may also vary when the number of cuts increases, affecting the yield of essential oils (Shahhoseini, 2022).
Associated arthropods
The insects associated with lemon verbena plants were 78 individuals corresponding to 50 families and 7 orders; 57 phytophagous, 15 predators, 3 parasitoids, and 3 pollinators were identified. Characterized phytophagous insects include the foliage-eating insects especially of the order Coleoptera, family Chrysomelidae, and the genera Diabrotica, Colaspis, Epitrix, Systena, Altica, and Cerotoma. These insects were observed as leaf borers throughout the crop cycle (Fig. 2); the suckers were members of the order Hemiptera, and the mites were members of the group of scrapers.

Figure 2. Families of arthropods observed in the crop of A. citriodora. A-E, Coleoptera. A-B, Chrysomelidae; C-D, Coccinellidae; E, Cantharidae. F-L, Hemiptera. F, Tingidae; G-H, Miridae; I, Cercopidae; J, Cicadellidae; K, Ortheziidae; L, Aphididae. M-Q, Diptera. M-O, Syrphidae; P, Tephritidae; Q, Stratiomyidae. R, Orthoptera: Eumastacidae. S, Lepidoptera: Gelechioidea. T, Phasmatodea.
The chrysomelids, whose mouthparts are chewing, causes direct damage to the leaves of plants, including the families Verbenaceae, Asteraceae and Solanaceae (Kher et al., 2016), these are also insects common to different crops in the Colombian coffee region such as tropical foliage (Aristizabal et al., 2013), therefore their presence is not exclusive to lemon verbena plant, but they can be potential pests for the crop, taking into account that they were observed throughout the production cycle and their presence was associated with damage to the foliage.
The insects of the order Hemiptera do not consume the foliage directly, however they do affect the appearance and reduce the vigor of plants in the process of sucking sap from the phloem, causing reduction poor growth, leaf curl, yellowing and as potential virus vectors, although the latter have not been reported for the species, they may become a predisposing factor. Also, the mites cause secondary damage such as chlorosis, color changes and defoliation, reducing the quality of the fresh material (Rehaman et al., 2018), and in cases of high infestation, the death of the plant.
Direct damage caused by insects restricts the use of fresh foliage, and implies an energy expenditure due to the defense process, causing changes at the physiological level such as a decrease in the quality of tissues from successive feeding events (Havill and Raffa, 2000), or even limiting growth, becoming an impediment to the marketing of healthy foliage for the production of quality essential oils (Amini et al., 2016).
As beneficial arthropod fauna, we found insects that reduce herbivory, among them two types of predators; those with chewing mouthparts, such as the order Coleoptera, family Coccinellidae, genus Cycloneda sp.; and of the order Hymenoptera, family Formicidae, genus Odonthomachus sp.; and those with sucking mouthparts such as assassin bugs of the order Hemiptera, family Reduviidae, genus Zelus sp., flies of the order Diptera, family Syrphidae, genus Toxomerus sp., which also have different attack strategies and target organisms (Fig. 2). Members of the family Formicidae establish mutualistic relationships with plants by releasing toxic substances during attack and dissolve the hemolymph of prey in exchange for shelter and food (Arcila-Hernández et al., 2017), as well as trophobiosis with members of Hemiptera, although this interaction was not visualized during the study, it is possible that it could occur and that in addition to the implicit damage, the appearance of fungal pathogens due to the secretion of honeydew is favored.
Coccinellids are generalist predators of soft-bodied insects such as aphids and coccids (Aristizabal et al., 2013), and have been related with different species such as Aphis gossypii, Myzus persicae, Thrips tabaci, T. palmi; Bemisia tabaci; Spodoptera sp. eggs, mealy bugs (Milán, 2010), Coccidae, Pseudococcidae and juvenile stages of Lepidoptera (Milán et al., 2008). Additionally, it has been mentioned that its distribution is related to the availability of prey for which it also has a preference depending on the species (Elekcioglu, 2020). In this case and taking into account that the population of aphids observed was greater than that of coccids, it is likely that those species whose feeding habit is aphidophag are favored, representing a potential biocontrol factor in this type of agroecosystems that can eventually be reinforced by flies of the Shyrpidae family that in the larval state are consumers of aphids (Dunn et al., 2020) and that were also frequently observed visiting Aloysia plant’s.
In parasitoid insects, specimens of the order Hymenoptera such as the families Braconidae and Tachinidae were found; as with other beneficial insects, their presence is determined by the complexity of the ecosystem and the chemical information they receive from the host plant (Escobar-Escobar et al., 2022) and due to the simplification of the environment (Tooker et al., 2020), such as forest fragmentation, and the establishment of monocultures. On the contrary, what was observed may indicate a supply of potential hosts, because these species appeared when the plant was in the vegetative stage V4, where the highest number of phytophagous insects was observed (Fig. 1).
Among the pollinators, members of the order Hymenoptera, families Halictidae and Apidae were identified, confirming the qualities of the lemon verbena plant as a melliferous plant, energetically attractive to pollinators and predisposing to the appearance of beneficial and generalist organisms such as bees (Botero-Restrepo, 2011). The distribution of the family Halictidae is worldwide and its members are listed as pollinators of more than one hundred crops, including aromatic plants (Rajkumar and Dey, 2016). The presence of members of this family represents an interesting association, as some are pollen specialists and have adapted both in behavior and morphology to a specific range of host plants, which in turn are also closely related (Danforth et al., 2008).
On the other hand, the Apidae family is one of the most ecologically important groups of insects, pollinating wild plants and contributing to the natural balance of ecosystems. According to Arnold et al. (2021) its presence is related to the richness of species of trees, plants and herbs with flowers in the interaction zones; an aspect that was favored by the accompanying plants on the edges of the crop; but additionally, it would be reaffirming the beekeeping value of the lemon verbena, with flowers that provide good volume and quality of nectar; essential elements for the sustainability of the species and in turn represent a potential resource in conservation and pollination programs (Nascimento et al., 2014).
Furthermore, the presence of pollinators in an ecosystem is influenced by the direct and indirect services provided by the ecosystem (Arnold et al., 2021); such as seed dispersal and pollination (Crespo-Pérez et al., 2020), which in turn is related to climatic factors, plant phenology and the type and number of associations established between plants and insects (Cuartas-Hernández and Gómez-Murillo, 2015).
The order Coleoptera was the taxon most frequently, with 25% of the total number of specimens identified and although their role in pollination is not as evident as members of the order Hymenoptera, due to their abundance in nature it is presumed that about 90% of pollinated plants have associations with beetles (Wojcik, 2021); although the size and characteristics of the lemon verbena flowers reduce the possibility that these insects act as pollinators, further research is suggested to determine their participation in this association, which has been considered merely phytophagous.
In general, the presence of beneficial insects does not depend exclusively on agronomic management; the other important factors are supply of food and shelter in plants adjacent to the crop, the structure of the habitat, the complexity of the assemblage in terms of plant species and insect taxa condition the ecosystem services they can provide (Arnold et al., 2021). The plant also has an active role after the release of volatile compounds, following the process of herbivory or oviposition, which acts as an attractant for parasitoids and predators (Havill and Raffa, 2000; Fürstenberg-Hägg et al., 2013). This could be observed, since most of the beneficial species were associated with vegetative stages where the damage caused by phytophagous was also more evident.
Similarly, the presence and permanence of some predatory insect species in the crop were possibly influenced by the surrounding plants such as Mexican sunflower (Tithonia diversifolia Hemsl. A. Gray), crotalaria (Crotalaria sp. L.), guandul (Cajanus cajan L. Millsp.), and arboloco (Montanoa quadrangularis Schultz Bipontianus) which provided additional ecosystem services, favoring generalist entomofauna. In the specific case of T. diversifolia, this is a species that serves as a refuge for predatory insects such as wasps, beetles and hoverflies, which were also found in this case (Baideng et al., 2020).
However, a longer period of cultivation is probably required to favor the process of adaptation and conformation of a suitable habitat that support the increase of the beneficial population of insects, especially those that provide ecological services such as pest regulation. Their increase is favored, among other aspects, by management systems that include cover crops, and organic amendments (Adhikari and Menalled, 2020), which can play a significant role in the interactions between plant and insects due to the microbial increase in the rhizosphere, changing the nutritional value of plants and defense mechanisms, as well as attracting and maintaining natural enemies (Rowen et al., 2019; Adhikari and Menalled, 2020).
CONCLUSION
The study of the phenology of the Lemon verbena plants made it possible to identify different stages of plant development, establishing a relationship with the associated arthropods and the interactions generated between them, taking into account the times of appearance and the direct effect they exert on the plant. This information contributes to the promotion of management plans that promote the increase and establishment of beneficial arthropod fauna, as well as opportune harvesting moments where the species expresses its maximum potential, according to the target market.















