Introduction
Congenital urological anomalies present in 4.3 out of every 10 thousand newborns,1 appearing either isolated or associated with problems in other systems. 2 They present in a variety of forms, ranging from renal agenesis to a wide diversity of kidney and urinary tract malformations that can be incompatible with life, 3,4 and end up as spontaneous abortions or stillbirths in over half of the cases. 5
Some of the most important associated anomalies of urological malformations are congenital heart defects. 6 Recent findings4 suggest an overlapping genetic etiology for heart disease and urological anomalies. However, despite the well-known association, it is still unclear if all newborns with urological malformations should be screened for congenital heart defects. 3
This poor prognosis of congenital urological anomalies at birth is worse in developing nations due to the limited experience of physicians regarding antenatal and postnatal diagnoses. 3 The problem overlaps with the lack of access to high-quality prenatal imaging and accurate detection, which remains a limiting factor in middle- and low-income countries. 7,8
Currently, there is scarce information about large-scale multinational studies addressing this issue. More accurate data may benefit clinicians in managing the risk of patients with congenital urological anomalies who might have a concomitant congenital heart defect. The present study is on the association between congenital urological anomalies and cardiopathies.
Methods
Database Description
The Latin American Collaborative Study of Congenital Malformations (Estudio Colaborativo Latino Americano de Malformaciones Congénitas, ECLAMC, in Spanish) has collected data on congenital malformations since 1967 using a case-control model. A total of 287 centers from 12 countries in South America and the Caribbean have participated in the study.
Personnel previously trained on the detection of congenital malformations oversees case findings. The detection of cases and controls is performed daily following the same methodology. Once a case is identified, all recognized malformations are registered using the ECLAMC coding system. If the group of anomalies can be classified as a syndrome, the case is registered under the name of the syndrome or the association. For the present study, syndromes are named as follows: Eagle Barret syndrome, Potter sequence, Down syndrome, Edwards syndrome, and congenital adrenal hyperplasia. For more details about the ECLAMC methodology, please refer to a previous publication by Poletta et al. 9 For comparisons, we used a 1:1 case-control ratio in the present study.
Data Collection and Inclusion Criteria
After obtaining approval from the institutional review board, we conducted a retrospective review of the ECLAMC database. All reported cases between 1967 and 2019 with urological and genital anomalies were included for analysis. Out of the total genitourinary (GU) malformations registered in the database, a subgroup with associated congenital heart defects (CHDs) (GU + C) was created and analyzed as a separate group. All demographic data for the cases (GU + C and GU-C) and controls were collected. We included information about the anomaly following the ECLAMC manual and pregnancy data (gestational age at birth, birth weight, and prenatal diagnosis).
For cases that presented more than one GU anomaly, the most severe one or the one that would impact ability or survival the most was considered the primary defect. Prevalence rates were calculated based on these criteria. When a group of anomalies could be included as part of a syndrome or sequence, data were grouped for analysis rather than analyzing isolated anomalies. For this specificgroup of urological anomalies, the ECLAMC also collects information about congenital Wilms tumors, and we also analyzed these reported cases.
The ECLAMC database involves antenatal ultrasonographic data and confi rmatory imaging to identify upper urinary tract anomalies immediately after birth. All included cases of Down syndrome were confirmed by karyotype before being added to the database.
Statistical Analysis
The estimated prevalence was calculated based on all newborns registered in the ECLAMC database during the study period. We conducted a data analysis regarding frequencies, means, and variances. Odds ratios (ORs) were calculated using a 95% confidence interval (95% CI). The variable comparison was performed with the Chi-squared test and analysis of variance (ANOVA). For the statistical analysis we used the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, United States) software, version 27.0, considering values of p < 0.05 statistically significant.
Results
We screened 7,767,161 newborns during the study period, and a total of 17,834 (0.23%) genital and upper urinary tract anomalies were identified. Out of those, 11,458 (64.2%) were genital anomalies, and the remaining (6,376 (35.8%) were upper tract anomalies. A total of 633 (3.5%) cases of GU tract malformations and concomitant CHDs were recognized. The gender distribution in this group was of 394 male newborns (62.2%), 180 (28.4%) female newborns, and 59 (9.3%) with indeterminate gender at birth. The average birth weight was of 2,408.7g (±856 g) for cases of GU + C, of 2,960.2g (±613g) for cases of GU-C, and of 3,224.16g (±714g) for healthy controls (p < 0.000).
Regarding maternal age at delivery, we found that mothers of GU + C newborns were on average 27.9 (±7.4 years) years old, mothers od GU-C newborns were 26.2 (±6.6 years) years old, and mothers of healthy newborns were 25.3 (±6.4 years) years old (p< 0.000).
The distribution of registered CHDs associated with GU malformations is presented in Table 1. The analysis showed that subjects with upper urinary tract anomalies had a probability of having cardiomyopathy three times higher than that of those who did not have those anomalies (Table 2). Other identified risk factors were horseshoe kidney, imperforated anus, and Down syndrome (Table 2).
Table 1 Congenital heart defects present in patients with genitourinary tract anomalies
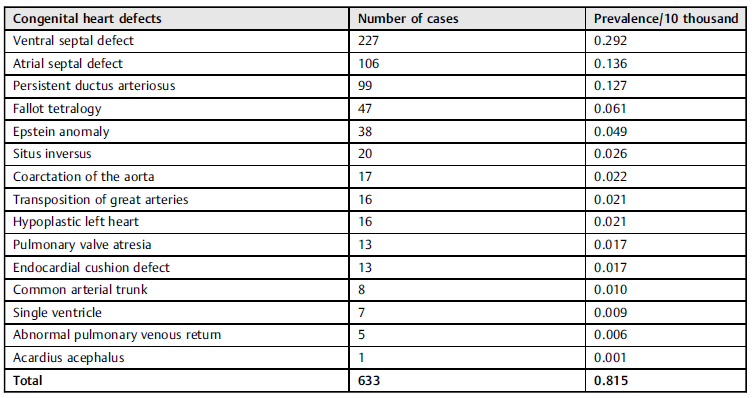
Note: *Non-significant relationships.
Table 2 Likelihood of the association between genitourinary tract anomalies and congenital heart defects
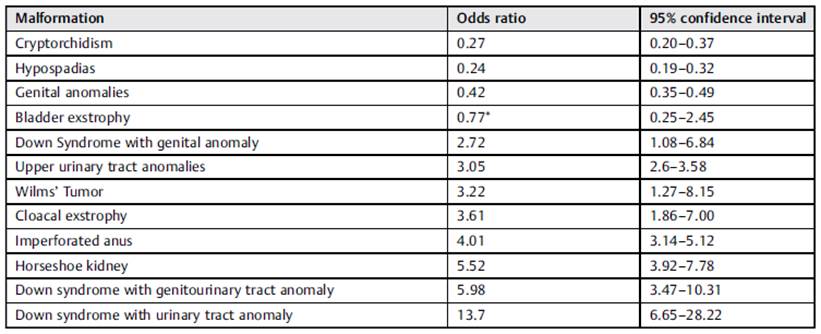
Note: Non-significant relationships.
The diagnosis was based on prenatal ultrasounds for 4,895 patients, and it was established at birth for 12,902 patients. In 37 cases, there was no data available on the moment in which the diagnosis was established. In total, 260 (41.1%) cases of GU + C were diagnosed antenatally. The first prenatal diagnosis was reported in 1994. For the first 10 years, a yearly average of 6.2 cases (GU + C) were detected. In the next decade (2005 to 2015), there was an increase to 11 per year.
Additionally, we found that patients with GU + Chadan average of 4.59 (±2.3) anomalies, while those with GU-C had an average of 1.53 (±1.58) (p < 0.000). There were 274 syndromic cases. The most common syndrome was Eagle Barret syndrome, with 140 cases and an estimated prevalence of 0.18/10 thousand newborns, followed by Potter sequence, with a prevalence of 0.08/10 thousand newborns. Wilms tumor at birth had a prevalence of 0.06/10 thousand newborns.
Out of the entire GU + C population, 16 cases also had Down Syndrome. The presence of upper urinary tract anomalies (the most common type of ventral septal defect followed by Epstein anomaly) and Down syndrome showed an OR of 13.7 (range: 6.65-28.22) for having a CHD.
Discussion
Congenital malformations are currently one of the leading causes of mortality and disability in the first year of life. 10 They are the cause of death in around 40% to 80% of the cases; 10 in Latin America, they are the leading root of morbidity and mortality in childhood. 11 Some of those congenital malformations can and will be treated; however, some of them will cause a lifelong impact, especially in low-and middle-income countries. 12 Therefore, it is essential to estimate the associations of those anomalies that can increase the likelihood of a poorer outcome.
A study by Li et al. 13 showed an association between heart defects and upper urinary tract anomalies in approximately 9% of the cases; our rate was slightly lower (3.5%). This difference may reflect that our investigation is a large-scale multinational study collecting data for 52 years, while the study by Li et al. 13 is hospital-based, with a shorter time-frame analyzed.
In a retrospective study, Jiang et al. 14 analyzed a database of 1,410 children with CHD from the Shanghai Children's Medical Center. They found 104 patients with abnormal urological systems, and the most common abnormality was hydronephrosis, followed by vesicoureteral reflux and duplication of the kidney and ureter. They found an overall prevalence of urological abnormalities of 7.4%.14 Furthermore, as they encountered a higher incidence of urological anomalies in patients with CHD, they suggested a routine examination for children with CHD to perform the early detection of urological malformations, 14 as it has been demonstrated that appropriate diagnosis and prompt management can reduce the mortality rates by 67% and the disease burden by 57% for patients born with heart defects. 15
A recent study by San Agustin et al. 16 can explain the increase in reports of the association between upper tract anomalies and heart defects. They found that 30% of heart defect-related mutations were also present in patients with renal malformations. The co-occurrence of urological anomalies and heart defects increases in cases of omphalocele, exstrophy, imperforated anus, and spina bifida (OEIS) complex. 17 Our results support these previously described findings with a four-time higher probability to have heart defects when OIES is present. These data are critical because the distribution of resources and improvements in the referral system become paramount for middle- and low-income healthcare systems. In our cohort, the association between Down Syndrome and GU + C was significant and kept the same trend of upper urinary tract anomalies associated with a higher risk when compared to genital ones in this subgroup of patients.
The present article serves as a guide to estimate the association of cardiomyopathies and urological malformations to help triage patients. Considering the limiting access to healthcare in Latin America, the present analysis can help prioritize referrals for urological patients with upper urinary tract malformations who are more likely to have a heart defect. 14 Additionally, it is essential to remember that the risk can be even higher in patients with horseshoe kidneys. The aforementioned data can be explained by the embryological vascular mechanism that has been proposed for this congenital defect. 6
The nature of a retrospective case-control study might suggest some limitations regarding the interpretation of the results. Additionally, we acknowledge that the present study may not reflect the current trends, but those before 1994, because cardiac defects and renal malformations are usually detected by prenatal ultrasonography. However, it is worth mentioning that, in previous studies, 8 we reported that the rate of prenatal detection of congenital urological anomalies was lower than 30% in Colombia. In the present analysis, the detection rate found was of 27.5%, which shows that prenatal detection has not been improved in our country.
Finally, the analysis of the cases identified by time groups did show an initial increase in reported cases. However, in the end, it showed a stable detection that reflected the actual capturing probability with this multinational congenital malformation surveillance system. The distribution of cardiac malformations follows expected and previously-published frequencies. 2
Conclusion
The association between CHDs and urological anomalies is higher in patients with complex congenital anomalies such as imperforate anus, cloacal exstrophy, and horseshoe kidney. Patients with urological anomalies and Down syndrome have the highest likelihood. Early detection and prompt referrals are needed. Low- and middle-income countries should prioritize the care provided to these patients at referral centers to improve survival and reduce mortality rates.














