Introduction
Leucaena leucocephala (leucaena) is a productive forage tree legume which contains the toxic non-protein amino acid mimosine. While this secondary plant compound can seriously affect animal health, in the case of ruminants, rumen microbes and endogenous plant hydrolase enzymes rapidly convert mimosine to hydroxypyridone (DHP), which is chronically rather than acutely toxic (Hegarty et al. 1964; Lowry et al. 1983). The widely published method of protection against DHP toxicity is via microbial degradation by a specialized rumen bacterium, Synergistes jonesii, strains of which, including the type strain (78-1), can degrade the mimosine-metabolite 3-hydroxy-4(1H)-pyridone (3,4-DHP) to 3-hydroxy-2(1H)-pyridone (2,3-DHP) and then to non-toxic by-products (Allison et al. 1992). Despite originally being considered non-ubiquitous (Jones 1994), S. jonesii is now considered a rumen microbe indigenous to the rumen (Halliday et al. 2013), albeit with discrete mutations or variants which have been detected in DNA gene sequences associated with different geographical locations. Variants of S. jonesii have been identified in ruminants in Indonesia (Padmanabha et al. 2014), which may account for the varying capacity of S. jonesii in certain animals to degrade DHP, and hence the historically perceived non-ubiquity of the microbe.
Seminal work on research into leucaena toxicity has demonstrated that functional S. jonesii can be rapidly and readily transferred from ‘protected’ animals to susceptible animals using rumen fluid inoculum (Jones and Lowry 1984). This methodology is effective in Australia with a cultured frozen inoculum available commercially, but may not be practical in eastern Indonesia, where vast distances separate ‘protected’ animals from those potentially susceptible to toxicity, including between islands. For effective microbial protection against leucaena toxicity to occur in susceptible animals, a practical method is required to successfully transfer rumen fluid from ‘protected’ to ‘unprotected’ ruminants, which may be in remote locations and often of a different species (e.g. goats, buffalo plus Bos indicus and Bos javanicus cattle).
Accordingly, this trial was conducted to investigate the feasibility and efficacy of inoculation of cattle and goats consuming high levels of leucaena in West Timor Island and excreting high levels of urinary DHP, i.e. not fully degrading all DHP. The inoculation consisted of rumen fluid from buffalo on nearby Sumba Island, which were consuming high leucaena diets but were excreting low levels of urinary DHP and therefore considered ‘protected’. As no commercial inoculum is currently available in Indonesia, it was also important to evaluate the practical feasibility of collecting, storing and transporting the rumen fluid anaerobically, with subsequent inoculation of smallholder animals, as a possible long-term solution for controlling DHP toxicity in eastern Indonesia.
Materials and Methods
The experiment was conducted on the islands of Sumba and West Timor in eastern Indonesia (Figure 1), over an 18 day period beginning in December 2012.
Donor animals were 2 female buffalo (Bubalus bubalis) from Melolo, Sumba. These buffalo were selected from a herd where S. jonesii had been previously detected (Halliday et al. 2014; Padmanabha et al. 2014) and were excreting low urinary concentrations of DHP (<10 mg DHP/L), while consuming diets containing >70% leucaena leaf and fine stems. Recipient animals were selected from areas known to be prone to DHP toxicity (Halliday et al. 2014), including: 3 kacang goats (Capra hircus) from Sumlili, Timor; and 4 Bali bulls (Bos javanicus) from Bone, Timor. All were consuming diets containing 50-100% leucaena and excreting high urinary concentrations of DHP (>900 mg DHP/L). Three Bali cattle (Bos javanicus, 2 bulls and 1 cow) from Tarus, Timor, previously consuming low levels of leucaena (<30% leucaena) in their diets, were also included. All animals were maintained on diets of 100% freshly cut leucaena leaf and fine stems throughout the monitoring period of 18 days. Recipient animals were randomly allocated to receive either the Sumba rumen fluid inoculum or a control treatment consisting of a water drench (Table 1).
Urinary DHP concentration was estimated visually following color development using the iron(III) chloride method (Figure 2) (Graham et al. 2014). Buffalo urine from donors was spot-sampled daily for 3 days prior to collection of rumen fluid, while recipient animals were monitored 2-4 times during the 8 days pre-inoculation, with further urine collections at 5 and 10 days post-inoculation. Methodology for hydrolysis of DHP to the free form was adjusted by omitting boiling for 1 hour (Graham et al. 2014), and instead allowing samples to hydrolyze over several days at ambient temperature (up to 37 ºC). During this period the color intensified, indicating hydrolysis of conjugated DHP consistent with the findings of Graham et al. (2014). Approximate concentrations of DHP, representative of free DHP after acid hydrolysis, were estimated based on color hue and intensity (Figure 2); the overwhelming majority of samples were dominated by the isomer 2,3-DHP, indicating at least partial degradation (from the isomer 3,4-DHP to 2,3-DHP) was occurring.
Rumen fluid for inoculation was collected via an orogastric tube (Graham et al. 2013), strained through muslin cloth, pooled, mixed and stored in a glass thermos and glass Schott® bottles. Animals were inoculated on the same day as collection, via an orogastric tube. All containers were initially flushed with rumen fluid, and young fresh ground leucaena leaves were added as a mimosine substrate. The bottles were kept at ~37 ºC and an anaerobic environment was maintained via the active fermentation of leucaena. Doses were: goats from Sumlili ‒ 100 mL; bulls from Bone ‒ 240 mL; and cattle from Tarus ‒ 150 mL. The trial was sanctioned under animal ethics approval # SAFS/144/11/ACIAR.
Repeated observations over time were analyzed using Minitab® statistical software 16 (©2010, Minitab Inc., State College, PA, USA) with a General Linear Model ANOVA using a split-plot design (with treatment as the main plot, time as the subplot and individual animals as a random factor, nested with treatment and location).
Results
Urinary DHP levels in recipient animals (in both the Sumba inoculum and control group) were high prior to inoculation (Table 2), at levels exceeding the considered safe threshold of 100 mg DHP/L (Dalzell et al. 2012).
Table 2 Mean (± s.e.) urinary DHP excretions from animals receiving either the inoculum of rumen fluid from Sumba buffalo or a control water drench as estimated by iron(III) chloride test.
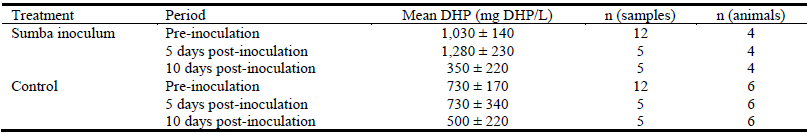
There were no significant (P = 0.50) differences in mean urinary DHP levels between animals dosed with the Sumba inoculum and those on the control treatment, either pre- or post-inoculation. Despite the lack of a treatment effect at 10 days post-inoculation, urinary DHP levels in all animals, while still high, had declined (most notably in the dosed animals), although they were not significantly different (P = 0.12) from pre-inoculation levels. The overwhelming majority of DHP in urine was excreted as the secondary isomer, 2,3-DHP. Mean urinary DHP levels for the 2 donor buffalo from Sumba (mean of 5 samples) were low (<10 mg DHP/L).
Treatment means are presented as an average of all animals receiving each treatment; animal species and location had no significant (P = 0.84) effect on DHP level. There was also no significant (P = 0.70) interaction effect between treatment and location.
Discussion
The results of this study indicate direct rumen fluid transfer was not effective in reducing urinary DHP levels in animals newly introduced to leucaena. This was evident as both a lack of difference between treated and control animals, and a lack of significant difference between pre- and post-inoculation DHP levels in the treated animals. Although there was an apparent decline in DHP levels in treated animals 10 days post-inoculation, this did not reach statistical significance, likely due to the small sample size and the high variability between animals. The main isomer excreted in animals was 2,3-DHP, indicative of partial degradation occurring; despite having originally been thought to be a transitory isomer, the presence of 2,3-DHP is now found to be the common isomer excreted.
This result contrasted with earlier reports where urinary DHP levels declined rapidly to safe levels following inoculation with rumen fluid, within 5 days in Indonesia (Jones and Lowry 1984) and within 3 days in Thailand (Palmer et al. 2010). However, while Jones and Lowry (1984) reported a “dramatic” decline in DHP excretion after inoculation, the 2 dosed goats in their experiment were already degrading 45-62% of ingested DHP prior to inoculation. This natural decline in DHP excretion and apparent inherent capacity to partially degrade ingested DHP was common in both these past, and the present, studies.
Although the scale of this study did not permit molecular analysis of rumen fluid samples, the donor buffalo belonged to the same herd which previously tested positive for S. jonesii. As the animals were consuming up to 100% leucaena diets, it was assumed that functional S. jonesii was present in the rumens of these donor animals owing to the negligible DHP levels in urine. In contrast, since mean urinary DHP levels in recipient animals, tested up to 4 times each pre-inoculation, were high (Table 2), exhibiting normal variation (most likely a function of the differential adaptation of individual animals to high leucaena diets within their first month on the diet) (Giles et al. 2013; Graham et al. 2013), we concluded that these animals were not fully ‘protected’ by rumen bacteria.
It is known that S. jonesii exists in the rumen in low populations, often below the limits of detection by nested PCR (<104-105 cells/mL) (Graham et al. 2013), and it is possible that the dosed inoculum did not increase ‘functional’ S. jonesii populations sufficiently to achieve protection. Although performed in vitro, the study of McSweeney et al. (1993) required 2 weeks for S. jonesii to establish a competitive population capable of degrading 3,4-DHP, quantified as abundance of S. jonesii RNA in a mixed-culture chemostat. Therefore, it seems our study should have continued for a longer duration. Doses in this study were limited by the volume of rumen fluid collected from the buffalo, and although dosage volumes were smaller than used in the work of Jones and Lowry (1984) (350 mL), they were greater than used by Palmer et al. (2010) (30 mL) (concentrations of organisms not measured). As S. jonesii was neither confirmed nor quantified in this study, it was also possible that the selected donor animals did not have effective S. jonesii populations. Possible alternative explanations for the low urinary DHP in donor buffalo include: degradation by microbes other than S. jonesii; the diurnal variation of urine volume affecting toxin concentration; and the possibility of a period of low leucaena levels in the diet.
Conclusions
Despite a natural decline in urinary DHP excretion in inoculated animals over time, differences failed to reach significance within the 10 day monitoring period (P>0.10). There was also no significant (P = 0.50) effect of inoculation, relative to the control animals. While limited by the length of monitoring, outcomes following inoculation were different from those originally reported over 30 years ago, using a similar methodology.
This study also highlighted the technical and logistical difficulties involved in collecting and transferring rumen fluid containing S. jonesii anaerobically as a method of inoculation against DHP toxicity, especially in multi-island countries, where supply-chain systems are limited. The methodology evaluated was not practically suitable for eastern Indonesia. The equipment and skills required to complete these actions are not currently available in the Indonesian Government livestock and extension services, and would require specialist training, the uptake of which would be problematical. If inoculation were to be viable, further work to develop suitable techniques is required, including exploring the possibility of transferring animals to allow the natural spread of rumen bacteria in a herd, if there was a need.
The apparent decline in urinary DHP levels in control animals indicated an inherent ability to adapt to DHP. Possible adaptation mechanisms include: the presence of an indigenous variant of S. jonesii or other DHP-degrading microbes; and metabolic detoxification of DHP by conjugation. Although the method of acid hydrolysis of DHP had been improved to optimize color development in iron(III) chloride reagent (Graham et al. 2014), it is acknowledged that there is a possibility that the apparent decline in measured DHP levels could be attributed to inconsistent hydrolysis among samples. Any remaining conjugated DHP would be unable to bind with Fe in solution, thus preventing the development of strength of color representative of the total DHP in urine. However, in the absence of chromatographic quantification of total DHP (be it free or conjugated), consistent hydrolysis is assumed.
Further work should aim to study: (a) the presence, functional capacity and relative contribution to degradation of DHP-substrate consumed of indigenous microbes within eastern Indonesia to better understand their role in initial degradation; and (b) the role of conjugation in detoxification of remaining undegraded DHP-substrate. At a practical level, finding an alternative control mechanism to eliminate the practical need for inoculation would greatly benefit these systems.