INTRODUCTION
Staphylococcus aureus is one of the major bacterial pathogens of medical interest [1]. Although it is estimated that approximately 20% of the general human population are persistently colonized with S. aureus, this microorganism can cause a wide variety of clinical complications ranging from self-limited superficial infections to severe bacteremia or pneumonia [2]. Various classes of antimicrobials are used for the treatment of these infections, such as β-lactams, macrolides, lincosamides and quinolones [3]. In addition, with the emergence of penicillin- and oxacillin-resistant strains since the 1960s, the use of vancomycin (glycopeptide) has also become common. Methicillin-resistant S. aureus (MRSA) strains, which are resistant to all β-lactams, were initially only detected in hospital settings [4]. However, since 1990, reports of resistant strains within the community have been described [5]. In the United States, the mortality rate from MRSA-associated infections outnumber those caused by HIV/AIDS and tuberculosis combined [6]. The colonization rate in American hospital settings is quite variable, and in some cases may affect up to 85% of patients [7]. In Brazil, 31% of S. aureus isolates from hospitalized patients are characterized as MRSA [8].
Macrolides and lincosamides are therapeutic options for the treatment of MRSA infection; however clinical failure of therapy has been reported when the strains harbor the erm gene. This gene encodes clindamycin-induced resistance and cross-resistance to erythromycin, conferring the macrolide-lincosamide-streptogramin B (MLS B ) resistance phenotype [9]. In general, exposure to subinhibitory antibiotic concentrations is related to MLSB resistance. In this context, wastewater is an important environment for the development of bacterial resistance as it harbors a complex bacterial community, receives residues of several antimicrobials and is considered a hotspot for gene exchange, including the exchange of genes that confer antimicrobial resistance (e.g., erm, bla ampo norA, acrABC, tetK, mecA and blaZ) [10, 11].
According to previous studies, the prevalence of S. aureus in wastewaters is low compared to clinical environments [12]. However, it should be highlighted that wastewater treatment plants (WWTPs) may be an important reservoir and source of MSLB-resistant S. aureus [13, 14]. Thus, considering the possibility of bacterial exchange between the clinical and environmental settings, investigation of the presence of resistant S. aureus strains in WWTPs is of great relevance as it may contribute to containing the spread of these microorganisms [13]. While many studies have investigated the resistance of enterobacteriaceae present in WWTPs, there is limited information on antibiotic-resistant S. aureus in this environment, which is of importance in developing countries [14]. Thus, we aimed to investigate the susceptibility of S. aureus isolates recovered from of a community WWTP in Brazil to several clinically important antimicrobials. In addition, the presence of clindamycin-induced resistance and penicillinase production was studied in isolates to determine the potential for this environment to act as a reservoir and source of MSLB- and penicillin-resistant S. aureus.
MATERIALS AND METHODS
Sample collection and recovery of Staphylococcus aureus
The area selected for this study was the city of Divinópolis (Minas Gerais), located in southeast Brazil (232 945 inhabitants). One liter of both raw sewage (RS) and effluent (EF) were collected from the Rio Pará WWTP (geographical coordinates: 20°08'20"S and 44°53'02"W) on June 8, 2015. The WWTP studded adopts the conventional activated sludge treatment system and receive domestic sewage generated by approximately 10% of the population from Divinópolis, been that their effluent is discharged in the Pará River. All samples were stored in sterilized polypropylene bottles and transported on ice to the laboratory within 2 h of collection. The sample collection was authorized by Companhia de Saneamento de Minas Gerais (Copasa), a publicly owned company responsible for the collection and treatment of sewage and water supply in the state of Minas Gerais (Brazil).
For the isolation ofS. aureus, 100 μL of RS and EF were plated directly onto mannitol salt agar (Labm, Brazil) in duplicate after being serially diluted (10-1 to 10-5) in a sterile saline solution 0.85% (NaCl). The plates were incubated at 37 °C for up to 48 h. After this incubation period, plates that had grown 20 to 200 colonies were selected for determination of the number of colonies forming units (CFU) per milliliter of RS and EF. Mannitol-fermenting colonies, which are yellow in color, were selected, inoculated in brain heart infusion (BHI) broth (Difco, India) and incubated at 37 °C for 24 h. Subsequently, the isolates were repeatedly streaked onto the same agar to check their purity. We also considered tests for catalase, coagulase and DNase, in addition to Gram staining, to confirm the species identification (S. aureus is positive for all these proves) [15]. The colonies isolated and identified as S. aureus were stored in nutrient broth containing 25% glycerol at -80 °C until further use.
Determination of antibiotic susceptibility profile
The antimicrobial susceptibility profile was determined by the disc diffusion method according to the recommendations of the Clinical Laboratory Standard Institute [16]. The following antimicrobials (DME Sensidisc, Brazil) were tested: β-lactams (penicillin, PEN), macrolides (erythromycin, ERT), lincosamides (clindamycin, CLN), and quinolones (ciprofloxacin, CIP; ofloxacin, OFX; norfloxacin, NOR). Cefoxitin disk (DME Sensidisc, Brazil) was used to predict the oxacillin susceptibility profile. Staphylococcus aureus ATCC 29213 was used as control.
Inducible clindamycin-resistance assay
The D-test was performed according to the CLSI (2017) [16] to phenotypically determine resistance to MSLB. Briefly, the antimicrobials clindamycin (2 μg) and erythromycin (15 μg) were placed at a distance of 15-26 mm on the surface of Mueller-Hinton agar (Alere, USA) which had been inoculated with each S. aureus isolate. The plates were then incubated at 35 ± 2 °C for 16-18 h. Verification of flattening in the erythromycin inhibition halo resembling the letter "D" indicates inducible resistance to clindamycin (figure 1). Staphylococcus aureus ATCC25923 and S. aureus ATCC29213 were used as controls.
Penicillinase production
Penicillinase production was investigated in all S. aureus isolates by the penicillin zone-edge method according to the CLSI (2017) [16]. This test is based on the appearance of the inhibition zone edge surrounding the penicillin G disk (DME Sensidisc, Brazil) after the disc-diffusion assay. The result was defined as negative when the appearance of the edge was fuzzy, resembling a "beach", and as positive when the edge was sharp like a "cliff". Staphylococcus aureus ATCC25923 and S. aureus ATCC29213 were used as controls.
RESULTS AND DISCUSSION
Methicillin-resistant S. aureus is one of the most prevalent multidrug-resistant microorganisms that cause infection in both the community and in health-care settings. Macrolides and lincosamides are therapeutic options for the treatment of MRSA-infections; however, resistance to these antibiotics has increased in recent years [17]. This phenomenon in S. aureus has rapidly emerged, mainly due to exposure to subinhibitory antibiotic concentrations combined with the acquisition of antibiotic-resistance genes, such as those of the erm family [18, 19]. Wastewater treatment plants combine these two factors, as well as having a nutrient-rich environment that favors microbial proliferation [10]. However, despite the importance of WWTPs in the dissemination of antimicrobial resistance, there is little available information concerning the impact of this environment on MSLB resistance in S. aureus. Furthermore, only few studies have evaluated the influence of domestic WWTPs on antimicrobial resistance in developing countries, and the dynamics of this phenomenon remain to be fully elucidated in these regions [10, 14]. Thus, in this study we aimed to evaluate the resistance profile as well as the phenotypic characteristics related to the clindamycin-induced resistance and penicillinase production in S. aureus isolated from a full-scale domestic WWTP in Brazil.
Microbiological analyses revealed 260 and 20 CFU/mL of S. aureus from RS and EF samples, respectively. In fact, several studies have revealed that, although there is often a high level of microbes present in the initial stages of wastewater treatment, microorganisms are either eliminated or reduced in final stage [12, 20]. Similar to this study, the sewage treatment employed in WWTPs in Spain (88.3%) [21] and Germany (99.9%) [22] also showed high clearance rates for Staphylococcus. The drastic reduction in the S. aureus population after wastewater treatment can, at least in part, be explained by the retention time of the effluent. According to Li et al. (2015) [23], the retention time of effluent has a negative effect on the survival of S. aureus because it disrupts important cell surface properties such as the zeta potential, hydrophobicity, and charge density.
A total of 35 different colonies (33 from RS and 2 from EF) were isolated on the mannitol agar, and these were included in the antimicrobial susceptibility tests and for phenotypic identification of resistance. As observed in table 1, in general the isolates showed high sensitivity to the antimicrobials tested except penicillin, which showed a considerable resistance rate (40.6%, 13/32) (figure 2). In accordance with our data, a high percentage of penicillin-resistant S. aureus in domestic WWTPs has also been found in Tunisia (100%) [24], Portugal (57.1%) [25] and Spain (40.62%) [21].
Table 1 Summary of resistance profile and identification phenotypic of inducible MSLB resistance and penicillinase production from S. aureus isolated in a full-scale domestic wastewater treatment plants (WWTP).
aS: susceptible; R: resistant; I: intermediate. bpositive test (+); negative test (-). NT: not tested; ERT: erythromycin; CLN: clindamycin; PEN: penicillin; CIP: ciprofloxacin; OFX: ofloxacin; NOR: norfloxacin; OXA: oxacillin.
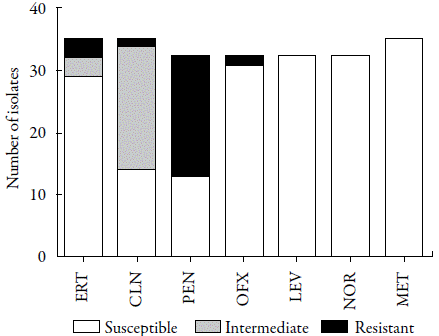
Figure 2 Susceptibility profile of Staphylococcus aureus isolated from a full-scale domestic wastewater treatment plants (WWTP). ERT: erythromycin; CLN: clindamycin; PEN: penicillin; OFX: ofloxacin; LEV: levofloxacin; NOR: norfloxacin; MET: methicillin.
Despite previous studies having indicated the presence of MRSA in WWTPs [14, 13, 26-28], our study did not identify any isolates with this phenotype (figure 2). Gram-negative bacteria are the predominant infectious agents in Latin America and the Caribbean, while Gram-positive bacteria are more frequent in the USA, Europe, and countries of the Pacific region [26]. Thus, it is expected that the selective pressure driving the spread of MRSA will be less frequent in Latin countries such as Brazil. Corroborating this hypothesis, MRSA isolates are most common in WWTPs in the USA [13, 14], Australia [27], Taiwan [28] and Spain [21].
One of the most interesting findings from the current study was the low level of resistance to quinolones observed among the isolates. These antibiotics are partially metabolized by humans and animals, remaining active in the aquatic environment, and they are not removed by the treatments normally performed in WWTPs [29]. Thus, quinolone-resistant strains can be easily found in this environment. In this study, of the S. aureus isolates tested, none showed resistance to levofloxacin and norfloxacin, and only one isolate from EF was resistant to ofloxacin (3.1%, 1/32). Similarly, most S. aureus isolates were sensitive to erythromycin (91.4%, 32/35) (figure 2). In the past decade, the clinical use of erythromycin has been limited and is often substituted with other antibiotics due to their better pharmacokinetic proprieties and fewer side effects [30]. Thus, the low rate of erythromycin resistance reported in this study can be explained by reduced selective pressure related to this antibiotic. In turn, although clindamycin-resistant S. aureus was uncommon in the WWTP studied, it should be noted that 20 isolates (57.1%) showed an intermediate level of susceptibility to this lincosamide, which highlights the possibility of the spread of resistance within this environment (figure 2). Goldstein et al. [13] reported that sewage represents an important route of dissemination of MLSB-resistant S. aureus, and the most common resistance gene related to this phenotype was identified to be ermC. The family of erythromycin ribosomal methylase (erm) genes encodes an adenine-specific N-methyl-transferase that methylates the 23S region of rRNA, conferring resistance to all macrolides, lincosamides, and streptogramin B [19].
Erythromycin-induced MSLB resistance was investigated by D-zone test. Two of 35 isolates tested, both derived from RS, were found to be D-test-positive (5.7%). This finding corroborates a study by Hess & Gallert [22], which reported that inducible MLSB resistance (D-test-positive) in S. aureus from sewage (14-19%) occurs at a lower frequency than constitutive MLSB resistance (62.2-75.5%). Penicillinase production was also investigated in 32 S. aureus isolates by the penicillin zone-edge method. A total of 27 isolates were found to produce penicillinase, although several of these (55.5%) were susceptible to penicillin. According to Kaase et al. [31] the penicillin zone-edge test is the most sensitive phenotypic method for penicillinase detection, but some species that not showed genetic determinants to this beta-lactamase, might have positive result in test. Thus, the inconsistencies between the findings in this study highlight the need to confirm, by molecular methods, whether blaZ gene is present in the positive isolates.
In summary, MRSA isolates were absent, and we found a reduced rate of resistance to erythromycin in full-scale domestic WWTPs studded. The high frequency of resistance to penicillin, in turn, suggests the indiscriminate use of this antibiotic in the region of station. In addition, the high rate of intermediate sensitivity to clindamycin among the isolates suggests that domestic sewage can contribute to the advancement of MLSB-resistant S. aureus. However, future studies should be performed to better understand the dynamics of this phenomenon, especially in WWTPs from other regions of Brazil.















