Introduction
Tuberculosis (TB), caused by the bacteria Mycobacterium tuberculosis, is a major public health problem that annually kills approximately 3 million people worldwide. It is worsened if there is co-infection with HIV 1. Approximately, one third of the world's population is infected with latent TB 1 , 2. The therapy against tuberculosis is based on the use of combined drugs such as rifampicin, isoniazid (INH), and pyrazinamide, which are also employed as components of currently applied multidrug therapy of TB 3. Generally, MDR-TB includes the strains resistant to at least isoniazid and rifampin, considered as the more efficient first-line drugs against TB. The occurrence of mutations within several genes is responsible for the MDR-phenotype. For example, in the case of INH resistance, mutations within katG, inhA, ahpC, kasA, genes are involved 4.
Despite the global availability of drugs against tuberculosis, there are still some issues to address: the long duration of the treatment, the serious side effects, and the development of multidrug-resistant strains 5,6. This reveals the urgent need to identify novel, safe, and effective candidates for the determination of an optimized and efficient drug against the M. tuberculosis.
Over the last decade, there has been extensive research on antibacterial compounds from natural products, since they are considered to be the major source of active metabolites. These may provide lead structures for the development of new drugs 7, for example, usnic acid (UA) appears to be a promising medical application. Usnic acid is a yellowish, highly functionalized dibenzofuran metabolite found in various lichen genera distributed in species of Cladonia, Usnea, Lecanora, Ramalina, Evernia and Parmotrema. It can exist as (+) and (-) enantiomers, but most of its biological activity is attributed to the (+) enantiomer 8 , 9. Usnic acid displays a wide range of multiple biological effects, including: anti-bacterial, anti-parasitic, anti-viral, anti-mycotic, anti-protozoal, anti-proliferative, anti-inflammatory, anti-pyretic, and citotoxicity against human cancer cell lines 10 , 11 ).
Furthermore, earlier studies reveal that (+)-usnic acid exhibits activity against M. tuberculosis, showing a MIC value of 32 μg/mL, which was not considered potent enough 12. Considering the fact that the functional groups found within the molecule make of usnic acid a good target for structural modifications 2 ), a reaction between UA and INH was suggested in order to evaluate the enhancement or not of its activity against M. tuberculosis.
In this context, this research presents the synthesis and characterization of an acyl-hydrazone (2) [(R,E)-N'-(1-(6-acetyl-3,7,9-trihydroxy-8,9b-dimethyl-1-oxo-1,9b-dihydrodibenzo [b,d]furan-2-yl)ethylidene)isonicotinohydrazide], obtained from a condensation reaction between usnic acid (1) and isoniazid, and the study of its anti-mycobacterial activity into M. tuberculosis strains.
Materials and methods
In general, all solvents and reagents (Acros Organics or Sigma Aldrich) were used without prior purification. The isolation and purification were monitored by thin layer chromatography (TLC) on silica gel plate (Merck 60 F254), preparative chromatography (Merck 60 F254), and by column chromatography using silica gel (Gel 60 silica particle size 0.063-0.200 mm).
Compounds 1 and 2 were analyzed and characterized in CDCl3 and DMSO-d6, respectively, by 1H-NMR 300 MHz, 1H-NMR 600 MHz and 13C-NMR 150 MHz with a Varian Unity Inova AS600 spectrometer. The notations used for the spectral analysis are: s (singlet), d (doublet), m (multiplet). Chemical shifts are reported in δ (ppm) using tetramethylsilane (TMS) as the internal standard. The determination of the exact molecular weights was performed by high resolution mass spectrometry (HRMS) on an Agilent Technologies Model 6210 LC-MSD-TOF (Time-of-Flight mass spectrometer) instrument. The molecular ions were protonated [M+H]+ or [M-H]-for the confirmation of their empirical formula. Infrared spectra were recorded on a JASCO FT/IR-410 spectrophotometer. The optical rotation was measured using a JASCO P-1010 polarimeter.
The M. tuberculosis isolates: H37Rv, TB DM97, and MDR DM1098 were obtained from Cayetano Heredia University Hospital. Their suspensions were prepared in 10% (v/v) Tween 80 (Sigma Chemical Co., St. Louis, Mo.) so that their turbidities matched that of the McFarland N°1 turbidity standard (approximately 3 x 107 CFU/ mL). Therefore, suspensions were diluted 1:25 in 7H9 broth (4.7 g of Middlebrook 7H9 broth base [Difco, Detroit, Mich.], 20 mL of 10% glycerol, 1 g of Bacto Casitone [Difco], 880 mL of distilled water, 100 mL of oleic acid, albumin, dextrose, and catalase [Remel, Lenexa, Kans.]) 13 , 14 ).
Extraction, isolation, and elucidation of usnic acid
The branched thallus of Evernia prunastri were collected at Porcon farm, located 30 km from the city of Cajamarca-Peru and identified by Dr. Luis Dávila of National University of Cajamarca, Cajamarca, Peru.
The branched dried thallus of Evernia prunastri (100 g) was crushed into a powder and extracted with an increasing polarity order of solvents using hexane (72 h (3x)), ethyl ether (72 h (x2)), chloroform (72 h (2x)), ethyl acetate (72 h (x2)), methanol (72 h (3x)), and water (72 h (3x)) at room temperature. The ethyl ether extract (4.8 g) was separated using repeatedly flash column chromatography with hexane/ethyl acetate (7:3) and hexane/toluene/ ethyl acetate (85:5:15), obtaining four fractions A, B, C and D. Fraction D was cooled at room temperature then filtered whereby a precipitate was obtained as a greenish yellow solid. This precipitate was separated out and recrystallized in ethanol 99% (v/v), from which the major compound was obtained as yellow crystals and identified as usnic acid (2.8 g), confirmed by 1H-NMR and 13C-NMR spectrums and by comparison with reported data 12 ).
Synthesis of acyl-hydrazone obtained from usnic acid and isoniazid
Usnic acid (500 mg, 1.45 mmol, 1 eq) and isoniazid (250 mg, 1.74 mmol, 1.2 eq) were added to a solution of 50 mL of absolute ethanol. The solution was stirred and heated at reflux for 12 h. The solution was cooled at room temperature and stored in a refrigerator for two days. The precipitate formed was filtered and dried in an oven at 25 °C for 24 h. The final product (acyl-hydrazone) was obtained as orange crystals 15 ).
Biological activity evaluation
The activity of usnic acid, isoniazid, and their acyl-hydrazone against susceptible (H37Rv), resistant wild type (DM 97) and multidrug resistance (DM 1098) M. tuberculosis strains were tested by tetrazolium microplate assay (TEMA), which uses tetrazoliumbromide [3-(4,5-dimethylthiazol-2-yl)-2,5diphenyl-tetrazolium bromide] (Aldrich Chemical Co., Milwaukee, Wis.) as a rapid, simple, and low-cost qualitative method to determine the antibiotic susceptibility 11 ). The assay was performed in duplicate (two plates). The procedure is described below 13,14 ).
Preparation of culture inoculum
Cultivation of M. tuberculosis was performed in Middlebrook 7H9 broth during four weeks. The inoculation of the strains was achieved by using a sterile loop into a glass bead tube with 100 μL of mix-Tween and mixed in a vortex for 2 min. Three mL of 10% (w/w) Tween 80 was added and stirred in a vortex for 20 s. The supernatant was transferred to a glass tube without beads. The turbidity was adjusted to McFarland N°1 with a mix-Tween solution. The McFarland N°1 strain was combined with Middlebrook 7H9 broth medium (12 mL of 7H9 + 0.5 mL of the strain) and diluted 1:25 in a Falcon tube.
Plate preparation and inoculation of drug and strain
Sterile water (200 μL) was added to all outer wells of sterile 96-well plates (Falcon 3072; Becton Dickinson, Lincoln Park, N. J.). Middlebrook 7H9 broth (100 μL) was added to the wells in rows B to O in columns 3 to 11 (labeled as commercially stamped on the plates). Drugs solutions (usnic acid, isoniazid and their acyl-hydrazone) (100 μL) were added to wells in columns 1 and 2; each two rows correspond to a different drug solution.
By using a multichannel pipette, 100 μL of solution was transferred from column 2 to column 3. The drug solutions were serially diluted 1:2 in consecutive columns through column 10, where 100 μL of excess medium was discarded. Final drug concentration ranges were 64 to 0.12 μg/mL. 100 μL of the diluted 1:25 strain was added to the wells in rows B to O in columns 1 to 11. The wells in column 11 served as inoculum-only controls. The plates were sealed with parafilm and then incubated at 37 °C for five days. Fifty microliters of a 1:1 mixture of the tetrazolium-Tween 80 mixture was added to well B11. The plates were reincubated at 37 °C for 12 h.
The following day, if well B11 turned purple, tetrazolium-Tween 80 was added to all wells and the color was recorded at 24 h. If well B11 remained yellow, the plates were incubated for another 24 h, after which tetrazolium-Tween 80 solution was added to well C11 before the plate was incubated for another 24 h. If well C11 remained yellow, incubation was continued and tetrazolium-Tween 80 solution was added to wells D11, E11, F11, and O11 on days 9, 11, 13, and 15, respectively. A yellow color in the well was interpreted as no growth, and a purple color was scored as growth. The MIC was defined as the lowest drug concentration, which prevented a color change from yellow to purple.
Results and discussion
Extraction, isolation and elucidation of usnic acid
Usnic acid (2.8 g) was obtained as yellow crystals from dried thallus of lichen Evernia prunastri (100 g), using repeatedly flash column chromatography with hexane ethyl acetate (7:3) and hexane toluene ethyl acetate (85:5:15). The IR spectrum showed bands at 2929 (CH-stretching), 1687 (elongation of the carbonyl group), 1628 (C=C, enol ether), 1540, 1455, 1421 cm-1 (aromatic ring). It should be mentioned that a wide band was present between 3500 and 2600 cm-1, due to the many hydrogen bonds present in the solid state.
The 1H-NMR spectrum shows characteristic signals at δ 13.31 and 8 11.02 ppm corresponding to the hydroxyl groups C8-OH and C10-OH respectively; δ 5.97 ppm corresponding to the H-4 from the double bond; finally δ 2.67, 2.66, 2.10; and 1.75 ppm corresponding to the methyl substituents. The hydroxyl group C3-OH appears at 18.84 ppm (Figure 1).
Usnic Acid: [α]D 25 (c = 0.7, CHCl3) = +451. Rf = 0.32 (hexane/ ethyle acetate 7:3). 1 H-NMR (600 MHz, CDCl 3 ): δ = 13.31 (s, 1H, OH), 11.02 (s, 1H, OH), 5.97 (s, 1H, CCH),2.67 (s, 3H, CH3), 2.66 (s, 3H, CH3), 2.10 (s, 3H, CH3), 1.75 ppm (s, 3H,CH3). 13 C-NMR (75 MHz, CDCl 3): δ = 201.9 (CO), 200.4 (CO), 198.2 (CO), 191.8 (COH), 179.5, 164.0,157.6, 155.3, 109.4, 105.3, 104.1, 101.6, 98.5, 59.2 (C dibenzofuran), 32.3 (COCH3), 31.4 (COCH3), 28.1 (CH3), 7.7 ppm (CH3). ESI - -HRMS: m/z calculated for C18H15O7 [M - H]-: 343.102; found: 343.100.
Synthesis of acyl-hydrazone obtained from usnic acid and isoniazid
The synthesis of the acyl-hydrazone was obtained with an overall yield of 95% (1.38 mmol) as orange crystals (Figure 2). The IR spectrum showed bands at 3415 (tension due to N-H), 2925 (CH-stretching), 1706 (tension due to carbonyl group), 1630 (C=N; C=C, enol ether, aromatic ring), 1549, 1362, 1220 cm-1. It should be noted that the NH group at 3415 cm-1 mas the signals of the OH groups present in the molecule.
The 1H-NMR spectrum shows characteristic signals of usnic acid and the corresponding signals of the protons belonging to the pyridine group, which appear upfield (Figure 3). The signals at δ 14.80, δ 13.40, and 8 12.13 ppm correspond to the hydroxyl groups C3-OH, C+-OH, and C10-OH respectively; δ 8.84 ppm, H-22, and H-23; δ 7.93 ppm, H-21 and H-24; δ 5.95 ppm, H-4; δ 3.45, NH. Signals at δ 2.71, 2.65, 1.98 and 1.69 ppm correspond to the methyl substituents.
The comparison of 1H-NMR spectrum of usnic acid and acyl-hydrazone revealed that the chemical shift of C3-OH is due to the presence of the imine group in the structure; the presence of this amine group also influences the electron density of the methyl group (Me-15), which appears shifted to downfield (δ 2.68 to δ 2.71).
Acyl-hydrazone: [α]D 23 (c = 0.25, acetone) = +205,6. Rf = 0.35 (hexane/toluene/ ethyle acetate/methanol 4:1:5:1). 1 H-NMR(600 MHz, DMSO-d 6 ): δ = 14.80 (s, 1H, OH), 13.40 (s, 1H, OH), 12.13 (s, 1H, OH), 8.84 (m, 2H, 2CH), 7.93 (d, 2H, 2CH), 5.95 (s, 1H, CCH), 3.45 (m, 1H, NH), 2.71 (s, 3H, CH3), 2.65 (s, 3H, CH3), 1.98 (s, 3H,CH3), 1.69 ppm (s, 3H, CH3). 13 C-NMR (75 MHz, DMSO-d 6 ): δ = 201.0 (CO), 197.5 (CO), 188.7 (COH), 173.6 (CO), 170.3 (CCO), 163.4 (CO), 157.7 (CO), 155.9 (CN), 149.4 (CN)pyr, 122.2 (CCN)pyr, 106.5 (CCC), 105.2 (CCC), 102.4 (CCHC), 101.0 (CCC), 100.9 ppm (CCC). ESI + -HRMS: m/z calculated for C24H22N3O7 [M + H]+: 464.1452; found: 464.1475.
Biological activity against M. tuberculosis
The activity of isoniazid, usnic acid, and their acyl-hydrazone against susceptible (H37Rv), resistant wild type (DM 97) and multidrug resistances (DM 1098) M. tuberculosis strains were evaluated (Figure 4). The tetrazolium microplate assay was performed in duplicate (two rows) based on the methodology already mentioned (see Materials and methods part). The MIC values obtained by compound 1 provide a better prospect to be potentially active compared to compound 2, the results are shown in Table 1.
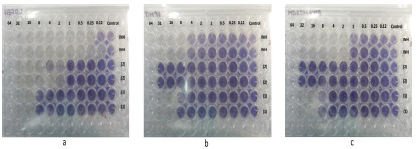
Figure 4 Biological activity of (1), (2) and isoniazid (INH) against (a) susceptible (H37Rv), (b) resistant wild type (DM 97), and (c) multi drug resistances (DM 1098) M. tuberculosis strains
Table 1 Antimycobacterial activity of INH, compound 1, and compound 2

aINH = Isoniazid. bCompound 1 = Usnic acid. cCompound 2 = Acyl-hydrazone
As it is shown in Table 1, acyl-hydrazone does not ameliorate the antimycobacterial activity of usnic acid for DM 97-resistant wild type, nor of H37Rv-susceptible strains, but, contrastingly, acyl-hydrazone showed an enhancement of its antimycobacterial activity, as compared to usnic acid on H37Rv-susceptible.
Conclusions
The synthesis of compound 2 (acyl-hydrazone) was achieved by a condensation reaction using commercial isoniazid and usnic acid, isolated from lichen Evernia prunastri, giving an overall yield of 95% (w/w). The isolation of usnic acid was performed using standard protocols of extractions and column chromatographic purifications. Their chemical structures were elucidated using 1D NMR spectroscopy and mass spectrometry, thus corroborating spectroscopic data reported in the literature 15 - 18.
According to the results obtained by TEMA method, usnic acid (1) exhibited MIC value of 16.0 μg/mL for each test of H37Rv, TB DM 97, and MDR DM 1098 strains. Instead, the corresponding acyl-hydrazone (2) exhibited MIC values of 2.0 μg/mL against H37Rv, and 64.0 μg /mL against resistant strain and multidrug resistance from M. tuberculosis.
The MIC values obtained by compound 1 provide a better prospect to be potentially active as compared to compound 2. However, both compounds exhibit less activity than isoniazid. Apparently, compound 2 does not meet initial expectations of increasing sensitivity on TB strains. However, these are preliminary in vitro results, and further in vivo studies should be carried out to confirm its antitubercular activity.

















