INTRODUCTION
Brazil has a prominent position in the world cattle industry, being considered the largest beef exporter (Menezes and Bacha, 2020). Thus, pastures are the main source of food for herds in the country, which has approximately 100 million cultivated ones (Moreira et al., 2014). Due to its low production cost, it represents the most practical and profitable form of animal feeding.
For pasture formation, the use of species of the genus Urochloa has stood out due to its rusticity (Lima et al., 2015). Among its various cultivars, marandu grass (Urochloa brizantha cv. Marandu syn. Brachiaria brizantha Hoechst Stapf cv. Marandu) stands out, with 60 million hectares of pastures formed in Brazil (Medica et al., 2017), whose preference is attributed to its good development throughout the year and excellent dry mass production (Bortoluzzi et al., 2017).
The implantation of marandu grass pastures in Brazil is subject to recurring problems with droughts during the period of germination and seedling emergence, compromising the establishment of the crop. Consequently, there is a restriction of water and nutrient availability, an increase in the number of weeds in the area, and a decrease in the support capacity of the pasture, resulting in the reduction of its perennity and of the profitability of the production system (Guimarães et al., 2018). Such problems become more pronounced in semi-arid regions, where evapotranspiration exceeds rainfall rates.
In this sense, water is one of the factors that most limit seed germination, whose restriction reduces the water potential of cells, triggering metabolic changes in plant physiology, such as a disordered production of reactive oxygen species (ROS) (Cechin et al., 2015). These molecules are highly reactive and, when in excess, become toxic and cause oxidative damage to several cellular constituents, such as enzyme denaturation, and carbohydrate oxidation, in addition to altering or inhibiting important metabolic pathways (Gill and Tuteja, 2010).
That said, ascorbic acid has been reported as a powerful antioxidant, with its exogenous application being recommended to fight stress, neutralizing the toxicity of ROS (Alak and Al-Sabagh, 2020). In this sense, its use has shown improvements in the germination of cowpea (Nunes et al., 2020), alfalfa (Salemi et al., 2019), and sunflower seeds (Fatemi, 2014) subjected to water restriction conditions. Moreover, it has been suggested that ascorbic acid has a bio-stimulating effect on germination, with improvements in seed vigor even under suitable water conditions, since it tends to synthesize enzymes responsible for redirecting reserve substances to the embryo during the germination process (Tommasi et al., 2001).
Despite research on seeds of different species subjected to treatment with antioxidants and water stress, the need to develop studies with marandu grass is highlighted. Considering the great relevance of this forage in the country and rainfall irregularity in several regions, the objective was to assess the effect of ascorbic acid dosages on the physiological potential of marandu grass seeds subjected to different water conditions.
MATERIAL AND METHODS
The experiment was conducted in the city of Janaúba, located in the northern region of Minas Gerais state (15° 49' S and 43° 16' W), from February to April 2020. Urochloa brizantha cv. Marandu seeds produced in the municipality of Campo Grande, MS (2019 harvest), were used.
To establish the time to be used for conditioning the seeds, the imbibition curve was determined; the time over which the seeds completed phase II of the three-phase imbibition pattern, without, however, proceeding to phase III, characterized by cellular elongation and primary root emission (Santos et al., 2008), was defined.
Thus, to study the water absorption behavior of marandu grass seeds, four repetitions of 100 seeds were used, which were weighed on an analytical scale (0.0001 g), and left soaking over nine periods (0, 2, 4, 6, 8, 10, 12, 24 and 48 hours) in a beaker containing 60 mL of distilled water, and kept in a germinator at a temperature of 25 °C under constant exposure to light. To monitor the absorption progress, the seeds were weighed during the established periods, whose weighing results were expressed as a percentage of fresh mass increase. The data were subjected to analysis of variance and regression, with the estimates being assessed by the "t" test (p<0.05).
To assess the effect of ascorbic acid on the physiological potential of seeds subjected to different water conditions, a completely randomized experimental design was used in a 3x5 factorial scheme, composed of three osmotic potentials (0; -0.2 and -0.4 MPa) simulated with aqueous solutions of polyethylene glycol 6000 (PEG 6000) and five doses of exogenous ascorbic acid (0, 20, 40, 60 and 80 mM), with four repetitions of 50 seeds per treatment, totaling 60 experimental units.
The water content of the seeds was preliminarily determined by the drying oven method at 105 ± 3 °C for 24 hours, using four repetitions of 100 seeds, with the results being expressed as percentages (Brazil, 2009).
Then, the seeds were disinfected by immersion in 0.5% sodium hypochlorite solution (v/v) for 2 minutes, washed with distilled water, and left drying on paper towels under laboratory conditions (± 26 °C).
For seed conditioning, L-ascorbic acid (+) P.A. ACS (C6H8O6) with analytical purity > 99% was used. The seeds were left soaking in different dosages of ascorbic acid, for a period of 12 hours (according to the results shown in Fig. 1), at a temperature of 25 °C, with the containers wrapped in aluminum foil to avoid degradation upon exposure to light. Immediately after imbibition, the seeds were dried until reaching their initial water content, for subsequent test setup under different water conditions.
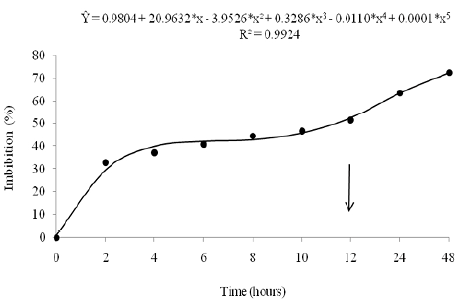
Figure 1 Imbibition curve in marandu grass seeds, expressed as a percentage of water absorbed about the initial weight of the seed, all subjected to different absorption periods. (*) significant at 5% probability by the "t" test.
To simulate the water conditions, the seeds were sown in a substrate moistened with PEG 6000 solutions at different levels of osmotic potentials. The amount of PEG 6000 to be added to obtain each water tension was determined through the equation proposed by Michel and Kaufmann (1973), corresponding to 9.59 and 13.93 g, for the -0.2 and -0.4 MPa potentials, respectively, with these amounts being diluted separately in 70 mL of distilled water. As for the 0 MPa potential (control), it consisted of distilled water only.
The germination test was run in plastic Gerbox-type containers (11.5 x 11.5 x 3.5 cm) with Germitest® paper as a substrate, moistened with the PEG 6000 solutions in an amount (mL) equivalent to 2.5 times the weight of the dry paper. The boxes containing the seeds were placed in a digital germinator, previously regulated at an alternating temperature of 20-35 °C, with a photoperiod of 8 hours of light at 35 °C and 16 hours of the dark at 20 °C (Brazil, 2009). The assessments consisted of counting the number of normal seedlings determined on the seventeenth day, with results being expressed as percentages. Seedlings presenting developed, proportional, and healthy essential structures (root system and aerial part) were considered normal.
Root protrusion was assessed 48 hours after the germination test was set up, which consisted of determining the number of seeds showing radicle emission with a minimum length of 2 mm, with the results being expressed as a percentage.
The first germination count consisted of recording the number of normal seedlings obtained on the seventh day after the start of the germination test, with the results being expressed as percentages (Brazil, 2009).
Simultaneously to the germination test, the germination speed index (GSI) was performed, which consisted of daily counting of the number of normal seedlings until the seventeenth day after sowing. At the end of the test, the GSI was obtained using the formula proposed by Maguire (1962).
On the seventeenth day, the root and aerial part lengths of ten seedlings/repetition considered normal were measured with the aid of a digital caliper, and the results were expressed as millimeters/seedling.
The data obtained were tested for assumptions of normality and homogeneity of variances using the Shapiro-Wilk and Barlett tests, respectively. After they wereattended to, the results were subjected to analysis of variance. Considering the two factors under study, the means from the osmotic potentials were compared using Tukey's test, while the means from the ascorbic acid doses were subjected to regression analysis. The estimates of the regression parameters were assessed by the "t" test (p<0.05), selecting the highest-degree models with biological behavior to explain the phenomenon. Statistical analysis was performed using Sisvar®-UFLA software versión 5.7 (Ferreira, 2011).
RESULTS
The imbibition periods were found to have a significative effect (p<0.05) on the fresh mass increment of marandu grass seeds, fitting the fifth-degree polynomial model equation. In the first hours, there was a rapid increase in the water content of the seeds, characterizing the 1st phase of imbibition (Fig. 1). After 4 hours there was stability in the hydration speed of the seeds until 12 hours of exposure, a peculiar behavior of the 2nd phase of imbibition. Phase III of the three-phase water absorption behavior started after 12 hours, with the seeds showing a resumption of growth in the hydration level until 48 hours of imbibition, culminating in the emission of the primary root.
Water content determination, as an initial procedure for assessing physiological potential, indicated that the seeds had an average moisture of 9.7%.
Based on the results obtained by assessing the physiological potential of the seeds, it was possible to observe a significant interaction between the different osmotic potentials and ascorbic acid dosages for all studied variables, after which the factors were unfolded.
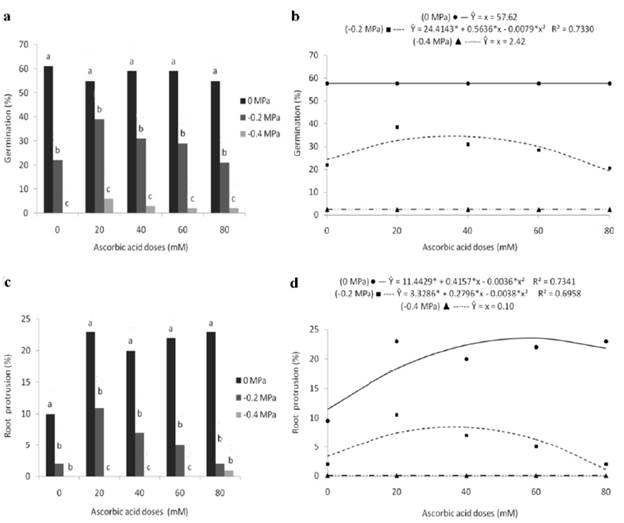
Figure 2 Germination (a, b) and root protrusion (c, d) of marandu grass seeds subjected to different osmotic potentials and exogenous application of ascorbic acid doses. Bars with the same letters, for each dose, do not differ statistically, by Tukey's test (p<0.05). (*) significant at 5% probability by the "t" test.
Analyzing the germinative capacity of the seeds (Fig. 2a) about the splitting of osmotic potentials, significant differences can be seen between the means obtained by the potentials in all ascorbic acid doses, in whichthe higher(61, 55, 59, 59 and 55%) and lowest (0, 6, 3, 2 and 2%) germination percentages were obtained by the control treatment (0 MPa) and by the exposure of the seeds to the -0.4 MPa osmotic potential, respectively, with intermediate germination values (22, 39, 31, 29 and 21%) at the -0.2 MPa potential.
Considering the effect of ascorbic acid doses, it can be seen that they influenced the germination of the seeds only when they were subjected to the -0.2 MPa potential, with results fitting a quadratic-behavior regression model (Fig. 2b). This way, as the ascorbic acid dose increased, the seed germination percentage rose to the 35.7 mM dose, when the highest percentage (34.5%) was verified. But, after that dose, germination decreased significantly, reaching a rate of 18.9% with the use of 80 mM of ascorbic acid.
Regarding root protrusion, it is possible to observe through the splitting of osmotic potentials, that, similarly to germination, in all doses evaluated, the control treatment (0 MPa) promoted statistically higher percentages (10, 23, 20, 22 and 23%) than those obtained by the other potentials (Fig. 2c). On the other hand, the lowest values (0, 0, 0, 0 and 1%) were obtained in the treatment with greater water restriction (-0.4 MPa) and, at the 0 and 80 mM ascorbic acid doses, the -0.2 and -0.4 MPa potentials showed statistically similar values.
When studying the unfolding of ascorbic acid levels,it was verified a significant influence on the seeds at the 0 and -0.2 MPa osmotic potentials, showing a quadratic behavior, with an increase in the percentage of root protrusion up to a certain dose, after which there was a decline (Fig. 2d). Analyzing the results obtained for the 0 MPa potential, the higher percentage (23.4%) was found at the 57.7 mM dose, while at the -0.2 MPa potential, the highest result (8.5%) was achieved at the 36.8 mM dose. For the respective osmotic potentials, the percentages were reduced to 21 and 1.4% with the use of the highest dose tested.
For the first germination count, by studying the effect of the potentials on each ascorbic acid dosage, greater results (17, 22, 26, 24 and 21%) were verified in seeds subjected to the adequate water condition (0 MPa).On the other hand, the -0.4 MPa osmotic potential showed a harmful effect, resulting in statistically lower percentages (0, 1, 0, 1 and 1%) at all doses (Fig. 3a). However, at the 80 mM dose, the percentages (3 and 1%) obtained by exposing the seeds to the -0.2 and -0.4 MPa potentials did not differ statistically.
About the ascorbic acid factor, a significant influence was found for the studied doses on the first count only in seeds exposed to the 0 and -0.2 MPa osmotic potential levels, in which the results were fitted to quadratic model equations (Fig. 3b). Considering the behavior obtained in the control treatment (0 MPa), rising percentages of normal seedlings are seen in the first germination count until the 46.4 mM dose, when the highest percentage (25.1%) was obtained, with a subsequent decrease that resulted in 20.5% at the 80 mM dose. In turn, the seeds exposed to stress conditions by osmotic potential -0.2 MPa presented higher results (7.3%) when using 34.9 mM of ascorbic acid, decreasing to 2.7% when the highest dose was used.
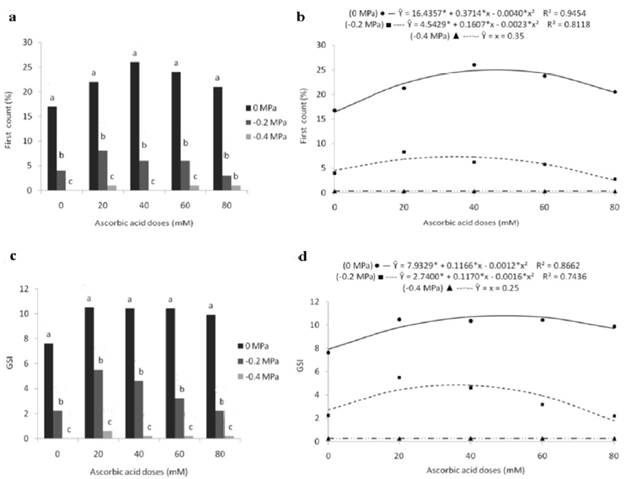
Figure 3 First germination count (a, b) and germination speed index - GSI (c, d) of marandu grass seeds subjected to different osmotic potentials and exogenous application of ascorbic acid doses. Bars with the same letters, for each dose, do not differ statistically, by Tukey's test (p<0.05). (*) significant at 5% probability by the "t" test.
The germination speed index was influenced by the osmotic potential,which when unfolded, was found in all ascorbic acid doses, that the control treatment (0 MPa) allowed statistically higher indexes (7.6; 10.5; 10.4; 10.4 and 9.9) about the other potentials(Fig. 3c). On the other hand, the lowest values (0.0; 0.6; 0.2; 0.2 and 0.2) were obtained in seeds subjected to water stress promoted by the -0.4 MPa potential. Intermediate results (2.2; 5.5; 4.6; 3.2 and 2.2) were found at the -0.2 MPa potential.
The effect of ascorbic acid levels on the germination speed index resulted in a behavior similar to root protrusion and first germination count, the results of which fit quadratic-model regression equations, with the dosages having significant effect only on the 0 and -0.2 MPa osmotic potentials (Fig. 3d).
In a suitable condition of water availability (0 MPa), the GSI stood at 7.9 in the control treatment (0 mM) and intensified through the exogenous application of ascorbic acid, reaching a maximum value of 10.8 at the 48.9 mM dose (Fig. 3d). From then on, when raising the dose, there was a decrease in the GSI, whose maximum level tested led to an index of 9.58. Regarding the -0.2 MPa potential, the maximum GSI was obtained by the seeds conditioned with 36.6 mM of ascorbic acid, which culminated in an index of 4.9 and reduced to 1.86 at the last dose.
For the 'root length' variable, considering the effect of osmotic potentials within each level of ascorbic acid, it was found that, in contrast to the statistically superior results (61.0; 62.6; 62.0; 64.4 and 58.1 mm) promoted by the control treatment (0 MPa), the -0.4 MPa potential provided seedlings with the shortest rootlengths(0.0; 28.3; 23.3; 15.9 and 10.4 mm).The -0.2 MPa potential, even though despite providing statistically similar results (30.6 and 23.3 mm) to those found at -0.4 MPa using treatment with 40 mM of ascorbic acid, in the other doses it differed, presenting intermediate values (Fig. 4a).
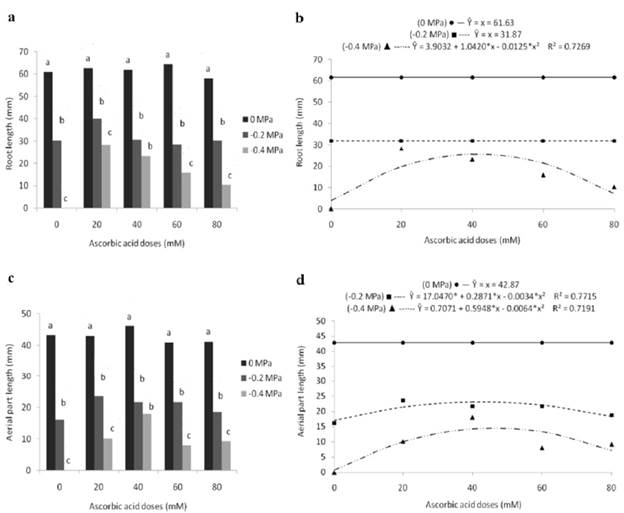
Figure 4 Root length (a, b) and aerial part length (c, d) of marandu grass seedlings from seeds subjected to different osmotic potentials and exogenous application of ascorbic acid doses. Bars with the same letters, for each dose, do not differ statistically, by Tukey's test (p<0.05). (*) significant at 5% probability by the "t" test.
Despite that, according to the unfolding of ascorbic acid doses, had a significant effect on the average root length only for the -0.4 MPa potential, presenting a quadratic equation (Fig. 4b). In this water condition, seedlings with lower root growth (3.90 mm) were obtained from seeds that did not receive the antioxidant. On the other hand, seeds conditioned with 41.7 mM of ascorbic acid originated seedlings with longer root lengths (25.6 mm). By raising the dose to 80 mM, the root growth was negatively affected, reaching 7.26 mm.
Similarly, to the other analyzed variables, the length of the aerial part was affected by the water restriction promoted by PEG 6000, whose unfolding of osmotic potentials revealed statistically inferior results (0.0; 10.2; 18.0; 8.0 and 9.2 mm) by exposingseeds to the -0.4 MPa osmotic potential, which, at the 40 mM dose, did not differ from the -0.2 MPa potential (Fig. 4c). The longest lengths (43.2; 43.0; 46.2; 40.9 and 41.1) resulted from the treatment without water restriction in the substrate (0 MPa).
However, the average length of the aerial part was significantly influenced by the ascorbic acid dosage for the -0.2 and -0.4 MPa potentials, resulting in a quadratic behavior (Fig. 4d). In their turn, greater lengths were obtained at the 42.2 and 46.5 mM doses, which corresponded to 23.1 and 14.5 mm, respectively, for the -0.2 and -0.4 MPa potentials. After obtaining the maximum length, there was a reduction in growth through high doses, resulting in seedlings with shoots measuring 18.26 and 7.33 mm, in the respective potentials at the time of the highest dosage tested.
DISCUSSION
The process of water absorption by the seeds followed the standard three-phase behavior proposed by Bewley et al. (2013), as seen in Fig. 1.
Considering the analysis of the seed soaking curve, it is verified that the quick absorption ofwater in the initial phase of soaking occurs due to the gradient of water potential between the seed and the external environment, that is, the water moves toward the seeds, which have lower water potential (Marcos-Filho, 2015). Then, the advance of the absorption process occurred slowly until completing phase II of the three-phase pattern,at which time the activation of pre-germinative metabolic processes occurs, in which enzymes, membranes, and organelles such as mitochondria become functional (Bewley et al., 2013).
Thus, the seed conditioning technique must allow all preparatory processes for germination to take place but prevent cell elongation with consequent emission of the primary root (Ibrahim, 2016). Therefore, the time of 12 hours to be used for seed conditioning was established, which stimulated metabolism during phases I and II of soaking, without advancing to phase III, characterized by root protrusión.
The water content of the seeds was found in the ideal range for the application of the tests, the percentage of which is situated within the limit (12%) established by Peske et al. (2012) for forage seeds, enabling consistent results in the evaluation of the physiological performance of the seeds.
Lower germination percentages obtained in seeds submitted to osmotic potentials -0.2 and -0.4 MPa denote stress promoted by the osmotic agent PEG 6000 regardless of the ascorbic acid dosage, which hindered seed hydration and, consequently, the manifestation of metabolic activities responsible for germination events. This fact evidences the sensitivity of marandu grass seeds to water deficiency during the initial stage of pasture establishment, causing a delay at the beginning of the germination process or a reduction in the germination rate with a consequent decrease in the initial stand of plants (Marcos-Filho, 2015). As a result, the total forage production is affected, minimizing the producer's financial return.
Compared to the control treatment (0 MPa), the conditioning of seeds with 35.7 mM of ascorbic acid allowed an increase of 41.3% in germination percentage, indicating the performance of ascorbic acid as a moderator of water stress. This is because ascorbic acid neutralizes the action of reactive oxygen species through the scavenging of radicals such as superoxide anion, hydroxyl radical, hydrogen peroxide, and singlet oxygen (Sucupira et al., 2012). Thus, the oxygen present in the medium is captured by ascorbic acid through chemical reactions forming stable compounds and consequently making it unavailable to propagate autoxidation (Rios, 2020).
On the other hand, doses higher than this promoted adverse effects on seeds, significantly reducing their germination percentage.This decrease with the use of high doses corroborates the analysis of Ishibashi and Iwaya-Inoue (2006), which also found that the application of high levels of ascorbic acid (50 and 100 mM) can interrupt or reduce the germination of wheat seeds.
The harmful effect of stress promoted by the osmotic agent was also verified on root protrusion, delaying the development of essential structures for the formation of a normal seedling. This behavior may be attributed to the reduction in seed metabolism due to the lower availability of water for the digestion of reserves and translocation of metabolized substances (Pereira and Lopes, 2011).
However, the stress promoted by the -0.2 MPa osmotic potential was mitigated by the conditioning of seeds with ascorbic acid, whose 36.8 mM dose represented an increase of 157.6% in the percentage of root protrusion about treatment without the use of the product (0 mM). Moreover, under adequate water conditions (0 MPa), ascorbic acid showed a biostimulant effect in the germination process up to the 57.7 mM dose, which in turn resulted in an increase of 105.3% about the control (0 mM dose).
The beneficial results of ascorbic acid on root protrusion during germination may be associated with it acting in the process of redirecting reserve substances to the embryo, stimulating the synthesis of enzymes involved in this process (Tommasi et al., 2001).
Considering the low percentages of normal seedlings obtained in the first count under water limitation conditions, the conditioning of seeds with ascorbic acid is a satisfactory technique to minimize the damage caused by osmotic stress (-0.2 MPa), allowing an increase of62.2% in the 34.9 mM dose about the result obtained with the absence of the product.
Considering that water deficit during the germination process generates oxidative stress, Ferro et al. (2016) highlight that ascorbic acid prevents the oxidation of several compounds. In this way, ascorbic acid molecules can oxidize before other molecules do, protecting such molecules against oxidation.
Regarding the 0 MPa osmotic potential, seeds conditioned with 46.4 mM of ascorbic acid obtained a 53% increase in germination in the first count about the control (0 mM), demonstrating that ascorbic acid not only acts as an antioxidant in stress conditions but also interacts during germination with phytohormones, as mentioned by Ye et al. (2012).
The formation of normal seedlings in the first count is associated with the vigor of the seeds, which is fundamental for a rapid and uniform emergence in the field. In this sense, the treatment of seeds with ascorbic acid increases its vigor, mainly due to the detoxication of "free radicals" (Nascimento, 2016), in addition to acting as a cofactor of antioxidant enzymes and other enzymes involved in seed metabolism during the germination process, among which deoxygenases stand out, which are responsible for the synthesis of gibberellin and abscisic acid (Smirnoff, 2018). Basra et al. (2006) also verified an increase in the vigor of rice seeds with the exogenous application of ascorbic acid about the treatment with the absence of the product.
For the germination speed index, regardless of the ascorbic acid dosage tested, the lowest values obtained in seeds submitted to water limitation show that germination occurred more slowly and irregularly. According to Antunes et al. (2011), the great molecular weight and the viscosity of polyethylene glycol make it difficult for water to pass through cell membranes, slowing down tissue hydration speed and oxygen diffusion, which means a longer time for the reorganization of the membranes and the progression of metabolic processes, with a consequent delay in the germination process.
Higher GSI values obtained with the exogenous application of ascorbic acid show greater vigor of the seeds, since vigorous seeds present higher speed in metabolic processes. These improvements in seed germination speed can be attributed to the action of ascorbic acid in several physiological processes, including metabolic changes in water deficit conditions to favor water availability (Khan et al., 2011).
Despite that, the excessive increase in the ascorbic acid level led to a reduction in its effectiveness caused by the likely phytotoxic effect of the solution on the seeds. In this sense, Dalberto et al. (2014), when working with wheat seeds, they found losses in the germination speed index through exogenous applications of 100 to 200 mM of ascorbic acid.
The limitation of root growth of seedlings formed in conditions of low water availability was minimized by the conditioning of seeds with ascorbic acid, whose 41.7 mM dose allowed an 86.15% increase in root length about the treatment that did not receive the product (0 mM). Thus, the capacity to develop extensive root systems is an aspect of great relevance, as it is associated with the potential of plants to obtain water and mineral nutrients from the soil.
On the other hand, after obtaining the maximum length, a reduction of 71.5% was observed in the 80 mM dose, revealing the need to establish the appropriate dosage, since excessive levels can produce an adverse effect both on root growth and in the other variables analyzed.
Considering the lower results obtained by water limitation during the development of the aerial part, Viçosi et al. (2017) explain that water deficit stress promotes a decrease in cellular water potential, resulting in a decrease in turgor pressure, which negatively affects cell expansion and stretching, as well as its metabolism, reflecting the reduction of shoot growth, as observed in this study.
The mitigating effect of the stress, promoted by ascorbic acid on the aerial part length, is conferred by its role in hormonal biosynthesis and antioxidant regeneration, in addition to being involved in the respiratory process, controlling cell division and expansion, processes that are directly related to formation and development of plant structures (Gallie, 2013). While assessing the conditioning of seeds of cowpea genotypes using ascorbic acid, Nunes et al. (2020) reported the development of more vigorous seedlings and a decrease in membrane damage caused by oxidative stress.
In summary, for the present study, ascorbic acid also favored the performance of marandu grass seeds and seedlings under water stress, which points to the antioxidant as a stress moderator, due to the protection they give them. The beneficial effect was also expressed by increases in root protrusion, first count, and seed germination speed index in adequate water conditions (0 MPa), which provides an alternative for treating seeds with the bio-stimulating effect of ascorbic acid on plant tissue.
Therefore, the use of ascorbic acid can be an important technology to be employed in the treatment of marandu grass seeds towards mitigating the deleterious effects of oxidative stress, commonly observed in conditions susceptible to water deficiency. In this circumstance, using seeds treated with ascorbic acid may result in better conditions for a more efficient pasture establishment. The adoption of this technology by the seed production industry, however, lacks information about the benefits obtained under practical pasture cultivation conditions, as well as about the industrial costs involved in the process, in monetary terms.
CONCLUSIONS
The physiological potential of marandu grass seeds is negatively affected by water stress induced by polyethylene glycol 6000, with deleterious effects being found at the -0.2 and -0.4 MPa potentials. Physiological conditioning with ascorbic acid at doses between 40 and 50 mM improves the physiological performance of the seeds and lessens the harmful effects caused by low water availability, providing tolerance to water stress.















