Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Ingeniería Universidad de Antioquia
Print version ISSN 0120-6230
Rev.fac.ing.univ. Antioquia no.75 Medellín Apr./June 2015
https://doi.org/10.17533/udea.redin.n75a15
ARTÍCULO ORIGINAL
DOI: 10.17533/udea.redin.n75a15
Influence of sodium chloride on the cloud point of polyethoxylate surfactants and estimation of Flory-Huggins model parameters
Influencia de cloruro de sodio en el punto de niebla de tensioactivos polietoxilados y estimación de los parámetros del modelo de Flory-Huggins
Alessandro Alisson de Lemos Araújo1, Eduardo Lins de Barros Neto1, Osvaldo Chiavone-Filho1, Edson Luiz Foletto2*
1 Department of Chemical Engineering, Federal University of Rio Grande do Norte. Av. Senador Salgado Filho, 3000. Natal, Brazil.
2 Department of Chemical Engineering, Federal University of Santa Maria. Av. Roraima, 1000. Santa Maria, Brazil.
* Corresponding author: Edson Luiz Foletto, e-mail: efoletto@gmail.com
(Received July 23, 2014; accepted April 05, 2015)
Abstract
In this study, the influence of NaCl on the cloud point of polyethoxylate surfactants from the family of lauryl alcohol polyethoxylates (C12EOn) and nonylphenol polyethoxylates (NPEOn) was investigated. Liquid-liquid equilibrium curves of the aforementioned aqueous surfactant systems with the presence of NaCl were plotted and thermodynamic parameters based on the Flory-Huggins model were obtained. The visual method was used to determine the cloud point. Solutions containing surfactant concentrations between 0.5 and 20% (by weight) and NaCl between 4.9 and 12.1% (by weight) were prepared. Salt had a salting-out effect, decreasing surfactant solubility in water. Furthermore, the cloud point decreased with an increase of NaCl concentration. The Flory-Huggins model satisfactorily described the experimental data for all surfactant + NaCl aqueous mixtures studied.
Keywords: Nonionic surfactant, cloud point, NaCl, Flory-Huggins model
Resumen
Este estudio investigó la influencia de NaCl sobre el punto de niebla de los tensioactivos polietoxilados de la familia de polietoxilatos de alcohol laurílico y polietoxilatos de nonilfenol. Se representaron gráficamente las curvas de equilibrio líquido-líquido de los sistemas acuosos con tensioactivos anteriormente mencionados con presencia de NaCl, surgiendo los parámetros termodinámicos basados en el modelo de Flory-Huggins. El uso del método visual determinó el punto de niebla. Se prepararon soluciones con concentraciones de surfactante entre 0,5 y 20% (por peso) y NaCl entre 4,9 y 12,1% (por peso). La sal tenía un efecto salino, disminuyendo la solubilidad surfactante en agua. Además, el punto de niebla disminuyó al aumentar la concentración de NaCl. El modelo de Flory-Huggins describió satisfactoriamente los datos experimentales para todas las misturas acuosas de surfactante + NaCl estudiadas.
Palabras clave: surfactante no iónico, punto de niebla, NaCl, modelo de Flory-Huggins
Introduction
The cloud point is a characteristic of nonionic surfactants and represents the broken interaction between the surfactant and water at a certain temperature. This occurs due to the decrease in affinity for water of hydrophilic groups that constitutes the surfactant head. This phenomenon is important because the use of nonionic surfactants in industrial processes is limited by cloud temperature (or cloud point), since there is a small amount of surfactants at a cloud temperature of 30 oC, such as Triton X-114 surfactants. The cloud point phenomenon causes the emergence of two liquid phases in equilibrium [1]. Thus, it is important to study additive compounds that affect the cloud point of nonionic surfactants, enabling their application at controlled temperatures and their use in extraction processes [2]. Various works about the effect of salts on the cloud point have been presented in the literature. The effect of added electrolytes on the cloud point of Triton X- 114 in the presence of ionic surfactants has been investigated [3]. The influence of chaotropic anions on the cloud point of surfactant octoxynol 9 (Triton X-100) has been studied [4]. The cloud-point diagram for Breox 50A-1000, an ethylene oxide–propylene oxide 50:50 (w/w) random copolymer, in water solution and the effects of electrolytes and surfactants on the cloud-point temperature have been determined [5]. Cloud point values of the aqueous two-phase micellar system formed by the nonionic surfactant Triton X-114 have been examined in water and in McIlvaine buffer (pH 6.5), either in the presence or absence of various salts at different concentrations [6]. Electrolytes and nonelectrolytes have been investigated in order to verify their influence on the cloud point of a series of nonionic surfactants of the poly(oxyethylene) ether type C12En (n = 6, 9 and 10) [7]. The infuence of various additives including inorganic salts, nonionic and ionic surfactants, water-soluble polymers and alcohols on the cloud points of three linear nonionic surfactants (Tergitol 15-S-7, Tergitol 15-S-9 and Neodol 25-7) has been investigated [8]. The effect of different additives such as electrolytes, urea, amphiphiles on the clouding behavior in zwitterionic surfactant/water systems has been determined [9]. Different cationic surfactants have been examined in order to determine the influence them on the cloud point of the nonionic surfactant Triton X- 100 in aqueous solutions [10]. However, to the best of our knowledge, works regarding the influence of NaCl on the cloud point of polyethoxylate surfactants from the family of lauryl alcohol polyethoxylates (C12EOn) (with degree of ethoxylation (n) of 8 and 9) and nonylphenol polyethoxylates (NPEOn) (with degree of ethoxylation (n) of 10 to 13) have not been yet investigated.
In this context, the influence of NaCl on the cloud point of polyethoxylate surfactants from the family of lauryl alcohol polyethoxylates (C12EOn) and nonylphenol polyethoxylates (NPEOn) was investigated. Liquid-liquid equilibrium curves of the aforementioned surfactants with the presence of NaCl were plotted and thermodynamic parameters based on the Flory-Huggins model, which described the binodal cloud point curve of the respective surfactants, were then determined.
Experimental
Materials and procedures
The nonionic surfactants used in this study were nonylphenol polyethoxylate (NPEOn), with ethoxylations 10, 11, 12 and 13 and lauryl alcohol polyethoxylate (C12EOn), with ethoxylations 8 and 9. NPEOn and C12EOn are represented by the following chemical structures:

A visual method was employed for determining the cloud point [6, 11-14]. In experimental methodology, solutions of 0.5% to 20% by weight of surfactant were prepared. Subsequently, these solutions were homogenized in magnetic stirrers. Then, they were placed in a jacketed cell under refrigeration and then using a magnetic bar to keep the homogenized solution, they were stirred. This experimental display was connected to a thermostatic bath. During the experiments the bath temperature was gradually increased and the cloud temperature of the solution was measured with a thermocouple (Salvterm 700K- SALCAS, ± 0.1 °C). For the purpose of confirming the cloud point, the solutions were cooled until they became clear and heated again to the temperature of the cloud, for the purpose of the measure confirmation. This procedure was repeated three times and only the mean values were reported. The maximum deviation observed was about 3.5 %. The NaCl solutions were prepared at concentrations of 4.9 and 12.1% by weight.
Flory-Huggins Model
Thermodynamic equilibrium is characterized by the difference in chemical potential in each phase, as Eq. (1):

Where 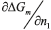 is a derivative from mixing Gibbs energy in relation to the number of moles of component 1, µ1m and µ1d represent the chemical potential of the most concentrated and diluted phases in relation to component 1, respectively.
is a derivative from mixing Gibbs energy in relation to the number of moles of component 1, µ1m and µ1d represent the chemical potential of the most concentrated and diluted phases in relation to component 1, respectively.
The chemical potential of the most concentrated phase is estimated by Eq. (2).

where ω123 is the parameter of the interaction between surfactant molecules (1), water (2) and NaCl (3) (represented by Eq. (3)), N corresponds to the number of surfactant aggregations, T is the temperature, R is the Universal Ideal Gas Constant, β is the number of water molecules and φm is the weight fraction of surfactant.

Substituting Eq. (3) in Eq. (2), results in Eq. (4).
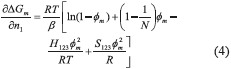
In equilibrium, the chemical potential for component ''1'' in the diluted phase is equal to the chemical potential in the most concentrated phase, µ1d = µ1m. Since the chemical potential is constant, the derivative from Gibbs free energy is equal to zero. Therefore, it results in Eq. (5).

where Tcalc is the temperature calculated by Flory-Huggins model, H123 is the enthalpy of the interaction between the surfactant, water and NaCl, S123 is the entropy of the interaction between the surfactant, water and NaCl, N is the number of surfactant aggregations and φm is the weight fraction of the solute.
Parameters H123, S123 and N from the Flory-Huggins model for each system was determined using a programming routine developed on the Scilab® platform, based on the modified Levenberg-Marquardt least squares numerical method. The routine used the objective function (O.F.) represented by Eq. (6):

The Figure 1 shows the calculation procedure for estimating the parameters of the Flory-Huggins model.
Results and discussion
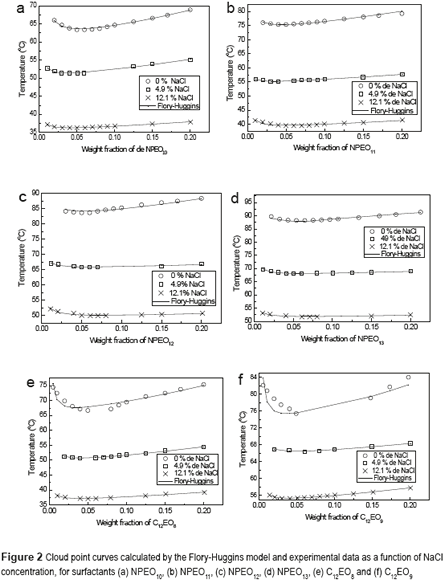
Figure 2 (a-f) shows a representation of the Flory-Huggins model and the experimental data for the cloud of surfactants NPEO10, NPEO11, NPEO12, NPEO13, C12EO8 and C12EO9, respectively, in the presence of NaCl. The curves depicted in Figure 2 (a-f) show that the Flory-Huggins model is a satisfactory representation of experimental data with the presence of sodium chloride. For all the surfactants, the presence of NaCl reduces the cloud point curve symmetrically, that is, the temperature variation is the same, regardless the salt concentration. This indicates that salt acts directly on water, where once surfactant affinity for the polar medium is reduced, any amount promotes formation of the surfactant-rich phase (coacervate). It can also be observed that for all the surfactants studied, the systems exhibit a lower critical solution temperature, which represents the region of liquid–liquid immiscibility; one phase is water-rich and the other is surfactant-rich. [15-17]. These results corroborate the evidence given by other researchers [18, 19]. The diminishing cloud point phenomenon is caused by the salting-out effect, which produces a decrease in surfactant solubilization in water. At concentrations of 4.9 % NaCl, the cloud point falls by about 12oC, and for concentrations of 12.1% NaCl, it decreases by 23.4oC, compared to the experimental condition without salt, for all surfactant concentrations studied. These results show that salt influences the cloud point of the surfactant. Thus, NaCl can be used in extraction processes to reduce the cloud point in order to save energy in this process.
Table 1 shows the Flory-Huggins parameters obtained for surfactants NPEO10, NPEO11, NPEO12, NPEO13, C12EO8 and C12EO9 in the presence of NaCl. Table 1 also demonstrates that, except for the surfactant C12EO9 both enthalpy (H123) and entropy (S123) increase proportionally with NaCl concentration, indicating that the addition of NaCl to the system increases the internal energy and, consequently, phase separation becomes easier; that is, interactions between the surfactant and water become increasingly smaller. Therefore, enthalpy and entropy increase by the increasing the salt concentration, and it is explained by the effect of the dissociation of the salt where the sodium chlorine ions unite with the water molecules forming strong bonds, thus increasing the enthalpy and entropy. These results confirm that the presence of NaCl promotes a salting-out effect on the surfactants.
Conclusions
The effect of sodium chloride (NaCl) on the cloud point of different polyethoxylate surfactants (NPEOn and C12En) was investigated. Binodal cloud point curves of the respective surfactants were determined by the Flory-Huggins model. In addition, thermodynamic parameters involved on the interactions between surfactant, water and NaCl (enthalpy and entropy) were obtained from the Flory-Huggins model. The cloud point curves of surfactants NPEOn and C12En in the presence of NaCl were satisfactorily described by the Flory-Huggins model. The presence of salt in the surfactant solution caused a drop in cloud temperature, and an increase in its concentration influence on the decrease of this cloud point.
References
1. I. Fischer, M. Franzreb. ''Direct Determination of the Composition of Aqueous Micellar Two-phase Systems (AMTPS) Using Potentiometric Titration – A Rapid Tool for Detergent-Based Bioseparation''. Coll. Surf. A: Physicochem. Eng. Aspects. Vol. 377. 2011. pp. 97-102. [ Links ]
2. K. Hung, B. Chen, L. Yu. ''Cloud-point Extraction of Selected Polycyclic Aromatic Hydrocarbons by Nonionic Surfactants''. Sep. Purif. Techn. Vol. 57. 2007. pp. 1-10. [ Links ]
3. T. Gu, P. Galera. ''Clouding of Triton X-114: The Effect of Added Electrolytes on the Cloud Point of Triton X-114 in the Presence of Ionic Surfactants''. Coll. Surf. A: Physicochem. Eng. Aspects. Vol. 104. 1995. pp. 307-312. [ Links ]
4. H. Schott. ''Effect of Inorganic Additives on Solutions of Nonionic Surfactants – XIV. Effect of Chaotropic Anions on the Cloud Point of Octoxynol 9 (Triton X-100)''. J. Coll. Interf. Sci. Vol. 189. 1997. pp. 117-122. [ Links ]
5. M. Cunha, F. Tjerneld, J. Cabral, M. Aires. ''Effect of Electrolytes and Surfactants on the Thermoseparation of an Ethylene Oxide–propylene Oxide Random Copolymer in Aqueous Solution''. J. Chromatogr. B. Vol. 711. 1998. pp. 53-60. [ Links ]
6. C. Santos, A. Lopes, A. Converti, A. Júnior, C. Rangel. ''Behavior of Triton X-114 Cloud Point in the Presence of Inorganic Electrolytes''. Fluid Phase Equil. Vol. 360. 2013. pp. 435-438. [ Links ]
7. K. Sharma, S. Patil, A. Rakshit. ''Study of the Cloud Point of C12En Nonionic Surfactants: Effect of Additives''. Coll. Surf. A: Physicochem. Eng. Aspects. Vol. 219. 2003. pp. 67-74. [ Links ]
8. J. Li, D. Bai, B. Chen. ''Effects of Additives on the Cloud Points of Selected Nonionic Linear Ethoxylated Alcohol Surfactants''. Coll. Surf. A: Physicochem. Eng. Aspects. Vol. 346. 2009. pp. 237-243. [ Links ]
9. P. Nilsson, W. Pacynko, G. Tiddy. ''Clouding in Zwitterionic Surfactant/Water Systems – The Influence of Additives on the Upper Consolute Loop of the Decyldimethylammonioethane Sulfatey/Water System''. Current Opinion Coll. Interf. Sci. Vol. 9. 2004. pp. 117-123. [ Links ]
10. H. Akbas, M. Boz, Ç. Batigöç. ''Study on Cloud Points of Triton X-100-cationic Gemini Surfactants Mixtures: A Spectroscopic Approach''. Spectrochim. Acta Part A. Vol. 75. 2010. pp. 671-677. [ Links ]
11. T. Inoue, T. Misono. ''Cloud Point Phenomena for POE-type Nonionic Surfactant in a Model Room Temperature Ionic Liquid''. J. Coll. Interf. Sci. Vol. 326. 2008. pp. 483-489. [ Links ]
12. T. Inoue, T Misono. ''Cloud Point Phenomena for POE-type Nonionic Surfactants in Imidazolium-based Ionic Liquids: Effect of Anion Species of Ionic Liquids on the Cloud Point''. J. Coll. Interf. Sci. Vol. 337. 2009. pp. 247-253. [ Links ]
13. T. Inoue, Y. Iwasaki. ''Cloud Point Phenomena of Polyoxyethylene-type Surfactants in Ionic Liquid Mixtures of EmimBF4 and HmimBF4''. J. Coll. Interf. Sci. Vol. 348. 2010. pp. 522-528. [ Links ]
14. A. Mohamed, R. Amina, V. Didier. ''Cloud-point Extraction of Bismuth (III) with Nonionic Surfactants in Aqueous Solutions''. Coll. Surf. A: Physicochem. Eng. Aspects. Vol. 375. 2011. pp. 169-177. [ Links ]
15. H. Radfarnia, V. Taghikhani, C. Ghotbi, M. Khoshkbarchi. ''A Free-volume Modification of GEM-QC to Correlate VLE and LLE in Polymer Solutions''. J. Chem. Thermodyn. Vol. 36. 2004. pp. 409-417. [ Links ]
16. E. Clark, J. Lipson. ''LCST and UCST Behavior in Polymer Solutions and Blends''. Polymer. Vol. 53. 2012. pp. 536-545. [ Links ]
17. T. Inoue, H. Ohmura, D. Murata. ''Cloud Point Temperature of Polyoxyethylene-type Nonionic''. Coll. Interf. Sci. Vol. 258. 2003. pp. 374-382. [ Links ]
18. K. Weckström, A. Papageorgiou. ''Lower Consolute Boundaries of the Nonionic Surfactant C8E5 in Aqueous Alkali Halide Solutions: An Approach to Reproduce the Effects of Alkali Halides on the Cloud-point Temperature''. J. Coll. Interf. Sci. Vol. 310. 2007. pp. 151-162. [ Links ]
19. N. Sato, M. Mori, H. ltabashi. ''Cloud Point Extraction of Cu (II) Using a Mixture of Triton X-100 and Dithizone with a Salting-out Effect and its Application to Visual Determination''. Talanta. Vol. 117. 2013. pp. 376-381. [ Links ]