Introduction
Northeastern Brazil accounts for 95% of the country's total melon production and meets the growing external demand since it is responsible for 97% of total exported melons. This is mainly due to the edaphoclimatic conditions and the technological packages used in the country that provide Brazil with up to three harvests a year. These conditions make Brazil an extremely competitive country worldwide when compared to European and Asian nations (Figueirêdo et al., 2017).
According to the classification of Robinson and DeckerWalters (1997), the melon (Cucumis melo L.) belongs to the Cucurbitaceae family and is divided into two botanical groups: 1) Inodorus, including yellow and frog skin, that are odorless and non-climacteric fruits, and 2) Cantaloupensis, including cantaloupe, gaul and charentais, that are climacteric fruits with marked aroma.
The type yellow is the most cultivated melon in Brazil because of its firm and thick skin that guarantees a longer shelf life and resistance to post-harvest transportation, facilitating exportation. Cantaloupes have become important melons in the domestic and international markets because of their characteristics of orange-colored pulp and high sugar content (°Brix). This melon is more aromatic and has light green and lacy skin, characteristics that directly influence consumer preference (Salviano et al., 2017). However, to have high productivity and guarantee competitiveness in the market, it is necessary to adjust the management and fertilization techniques to the edaphoclimatic conditions of each production region, such as the Pontal of Triângulo Mineiro (Minas Gerais State, Brazil) that has naturally poor soil and high annual mean temperatures (Sousa & Lobato, 2004).
Among the macronutrients, potassium plays an important role in numerous physiological processes that alter growth and, consequently, crop productivity. This nutrient regulates the opening of stomata, limiting water loss, and acts in the photosynthetic processes of plants (Zörb et al., 2014; Cavalcante et al., 2018), guaranteeing reproduction and fruit quality. Deficient potassium fertilization directly interferes with melon productivity, reducing the fruit size, lowering levels of soluble solids and ascorbic acid, changing the color and, consequently, reducing shelf life (Lester et al., 2010). Bardiviesso et al. (2015) observed that the application of 136.75 kg K2O ha-1 on melons increased fruit yield and significantly affected the concentration of soluble solids and pH, meeting the appropriate standards for fruit marketing.
Various sources of potassium used in agriculture have different points of deliquescence (POD) of salts that indicate the rate of absorption by plant tissues. Schönherr and Luber (2001) observed that this rate, in turn, was dependent on environmental conditions of temperature and humidity. These authors reported POD values from commonly used potassium sources: K2CO3, 44%; KCl, 86%; KNO3, 95%; and KH2PO4, 97%. These data can help producers make decisions about which fertilizer to use, depending on the edaphoclimatic conditions of the planting region.
However, different sources of potassium, due to their characteristics and mode of action on vegetal metabolism, can affect differently the production and quality of the melons. Thus, the objective of this study was to evaluate the effects of fertilization with various potassium sources, at planting and topdressing, on the physicochemical characteristics of cantaloupes cultivated in the Pontal of Triângulo Mineiro, Minas Gerais, Brazil.
Materials and methods
The study was conducted in the experimental area of the School Farm "Alípio Soares Barbosa" and at the Chemistry Laboratory of the Federal University of Triângulo Mineiro, Iturama Campus (UFTM/ITU), Minas Gerais, Brazil (19°43'41" S, 50°11'44" W, at an altitude of 425 m a.s.l.). The climate of the city of Iturama is classified, according to Köppen-Geiger, as Aw, with a rainy season from October to March and a dry season from April to September. The average annual rainfall is 1266 mm and the average temperature is 24.5°C.
Before planting, the soil was sampled from the 0-20 cm layer and chemically characterized (Van Raij & Quaggio,1983) as follows: pH (CaCl2) 4.8; organic matter: 24 g dm-3; P (resin) 47 mg dm-3; K: 2.1 mmolc dm-3; Ca: 22 mmolc dm-3; Mg: 8 mmolc dm-3; S: 12 mg dm-3; H+Al: 47 mmolc dm-3; sum of bases: 32.1 mmolc dm-3; cation exchange capacity: 79.1 mmolc dm-3; base saturation: 41%; B: 0.17 mg dm-3; Cu: 1.2 mg dm-3; Fe: 75 mg dm-3; Mn: 4.1 mg dm-3, and Zn: 3.2 mg dm-3.
The field was prepared under a conventional tillage system using a disk harrow for soil decompaction, and then a light harrowing to level off the ground and eliminate weeds. To correct the acidity of the soil, one month before the beginning of the experiment, the equivalent to approximately 4 t ha-1 of calcitic limestone (total relative neutralizing power = 85%) was applied, defined based on the soil analysis and following the recommendation of Van Raij et al. (1997).
For soil correction, 20 m3 ha-1 organic compost, 120 kg ha-1 P2O5 (single superphosphate) phosphorus and 250 kg ha-1 phonolite powder (8% K2O; 54% SiO2; 6.74% Na2O) were applied in two applications: the first 30 d and during the last 10 d before transplanting the seedlings. After transplanting, topdressing fertilization was performed with nitrogen (17g m-2 of N [urea]) and potassium (25 g m-2 of K2O), in four applications as follows: at the planting of seedlings and 15, 30, and 45 d after transplanting. A drip-tape irrigation system was used to irrigate the experimental area, using a nominal flow of 3.6 L h-1, at 60 kPa of service pressure, with emitters spaced at 0.30 m. The plants were irrigated throughout the cycle, always keeping the soil moisture above field capacity, initially in two 20-min daily shifts, and then three 20-min shifts, from the beginning of fruiting until harvest.
The experiment was conducted in a randomized complete block design with a factorial arrangement (2x4) of treatments (with or without sowing fertilization with Ekosil™ [8% K2O, 25% total silicon] and topdressing fertilization with potassium nitrate [PN; 12% N, 43% P2O5, 1°% S and 1°% Mg], potassium chloride [PC; 60% P2O5], potassium sulfate [PS; 51% K2O, 18% S], or Ekosil™ [EK; 8% K2O, 25% silicon]).
Ekosil™ (Yoorin Fertilizantes, Poços de Caldas, MG, Brazil) is a potassium fertilizer obtained by grinding silicate rocks of volcanic origin without using chemicals and has a saline index of 0.63. It has a residual effect with a gradual release of nutrients during the vegetative and productive cycle of plants. Thus, it causes fewer potassium losses due to leaching since the accompanying anion is the silicate. It is composed of 8% K2O and 25% total silicon.
The experimental area was composed of approximately 60 m2, divided into four blocks spaced 1.20 mx0.30 m (rows and plants, respectively), totaling 160 evaluated plants (five per treatment combination in each block). The melon hybrid used was the Torreon F1 from Top Seed (Agristar, Guimarânia, MG, Brazil) that produces oval fruits, with lacy peel, orange pulp, a small internal cavity, and an average mass of around 1,000 g. Sowing was carried out in expanded polystyrene trays in July 2019 and field planting was carried out in August 2019. The plants were managed vertically without pruning of branches or thinning of fruits and tutored on a bamboo spreader horizontal to the soil and tied to posts fixed in the ground. The harvest point was determined when the peduncle of the fruit showed the abscission layer and was easily detached from the plant.
After harvesting, the fruits were transported to the Chemistry Laboratory of the Federal University of Triângulo Mineiro for evaluation. The characteristics evaluated in the fruits were: 1) fresh fruit mass (FM), using a digital analytical scale (model S4202, Bel Equipamentos Analíticos, Piracicaba, Brazil) and expressed in grams; 2) content of soluble solids (SS), quantified with an aliquot extracted from the pulp after crushing by gauze compression and quantified using a palette refractometer (model PR-101, Atago, Tokyo, Japan), expressed in °Brix (AOAC, 1997); 3) titratable acidity (TA), using 10 g of crushed pulp, diluting it in 50 ml distilled water, titrating with 0.1 N NaOH and expressed in grams of citric acid 100 g-1 pulp (AOAC, 1997); 4) ratio (RA) between the levels of soluble solids and titratable acidity; 5) pH, using a digital pH meter (model PHS-3E, Shanghai Yoke Instrument, Shanghai, China); 6) carotenoid content (CA), and 7) chlorophyll content (CL), 2.0 g of each treatment were weighed directly into centrifuge tubes, then 18 ml 80% acetone was added, homogenized and centrifuged at 1,000 g for 5 min in a centrifuge (model 80-2B, Kubota Corporation, Daiki, Akagidai, Japan). The readings of the supernatant were performed using a spectrophotometer (model F-7100, Hitachi High-Tech Science Co, Tokyo, Japan) with absorbances of 663 nm (chlorophyll a), 646 nm (chlorophyll b), and 470 nm (carotenoids) and expressed in mg g-1 of pulp (Arnon, 1949).
An analysis of variance was performed using the Glimmix procedure of SAS version 9.4 (SAS Institute Inc., Cary, USA). Means were compared using the Tukey's test and the significance was declared at 5%.
Results
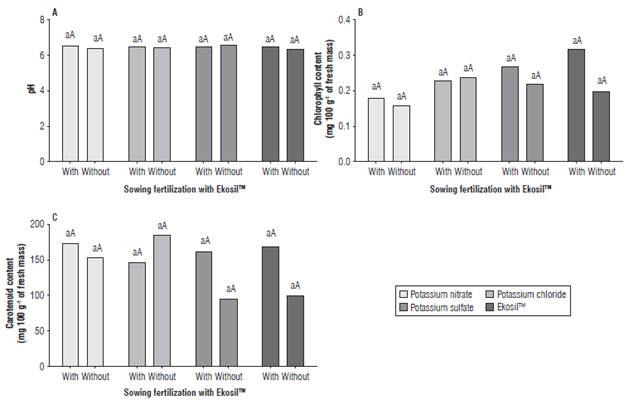
An interaction of sowing fertilization with Ekosil™ and topdressing potassium sources was observed for the variables FM (P = 0.005), SS (P<0.0001), TA (P = 0.0002) and RA (P<0.0001; Fig. 1) but no interactions were observed for the variables pH, CL, and CA (P>0.05; Fig. 2).
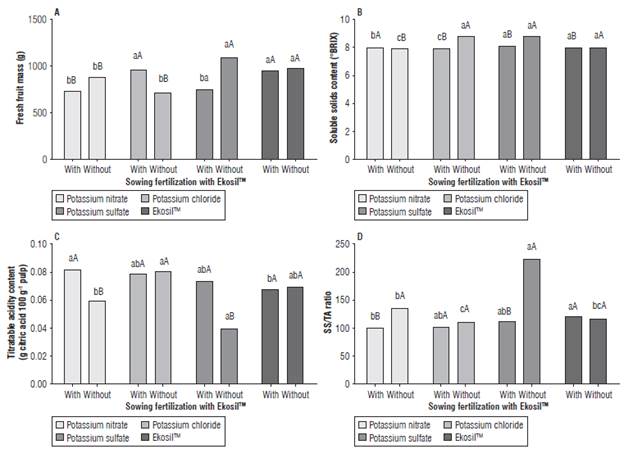
FIGURE 1 A) Fresh fruit mass; B) soluble solids content (SS); C) tltratable acidity content (ТА), and D) SS/ТА ratio of cantaloupe cultivated with or without sowing fertilization with Ekosil™ and topdressing potassium sources (potassium nitrate, potassium chloride, potassium sulfate or Ekosil™). Means followed by different capital letters differ from each other regarding the use of sowing fertilization with Ekosil™ according to the Tukey's test (P<0.05). Means followed by different lowercase letters differ from each other regarding the source of potassium, according to the Tukey's test (P<0.05).
When using sowing fertilization with Ekosil™, the top-dressing potassium sources PC and EK provided heavier fruits when compared to PN and PS that were similar. However, when not using Ekosil™ at sowing, the treatments with heavier fruits were PS and EK, followed by PN and PC. When evaluating data within each potassium source, differences in the use of Ekosil™ at sowing were observed for PC resulting in heavier fruits with sowing fertilization with Ekosil™ and for PS resulting in heavier fruits when not fertilizing with Ekosil™ at sowing (Fig. 1A).
The effect of topdressing potassium sources on SS was observed with or without fertilization with Ekosil™ at sowing. The topdressing PS provided greater SS content, followed by PN and EK (without any differences between them) and PC when the sowing fertilization with Ekosil™ was performed. The effects were different when not using Ekosil™ at sowing, with greater SS contents for treatments PC and PS and lower for EK followed by PN. Effects of sowing fertilization with Ekosil™ within each topdressing potassium source were observed. For PC and PS, the greater values were observed when not using sowing fertilization with Ekosil™, whereas for PN the opposite was observed, and for EK no difference was seen (Fig. IB).
The TA was altered by topdressing potassium sources and sowing fertilization with Ekosil™. The lowest ТА content was observed for EK and the highest for PN, with PS and PC showing intermediate values ranging from 0.074 to 0.079 g citric acid 100 g 1 when using sowing fertilization with Ekosil™. When not using Ekosil™ at sowing, the lowest TA content was observed in PS and the highest in PC, with PN and EK ranging from 0.060 to 0.070 g citric acid 100 g 1 . The treatments PN and PS were affected similarly, showing greater TA content averages when sowing fertilization with Ekosil™ was used. No effect of sowing fertilization was observed for either PC or EK (Fig. 1С).
Although representing the SS/ТА ratio, the results for RA were higher for EK and lower for PN when using sowing fertilization with Ekosil™. The results of this variable were greater for PS, followed by PN and EK (with similar values) and PC, when not using Ekosil™ at sowing (Fig. ID).
The pH, CL, and CA were unaffected by sowing fertilization with Ekosil™ and topdressing potassium sources, with averages of 6.49,0.23 mg g1 FM, and 149.0 mg g1 FM, respectively (Fig. 2A-C).
Discussion
The absorption of potassium by the roots is a highly efficient process for removing the nutrient from the soil, as long as there is an adequate amount of water for it to be transported via the xylem by mass flow (Römheld & Kirkby, 2010). The transport of nutrients by plants depends on the solubility of potassium sources, and this will influence the availability of this macronutrient for plants. Thus, in this experiment, it was seen that the PC and EK sources resulted in heavier fruits since the low solubility of the rock powder (Ekosil™) gradually provided nutrients to the plants that may result in larger fruits (Ehlers & Arruda, 2014). Feltrim (2010), evaluating fertilization with potassium nitrate and potassium chloride and spacing in watermelon, did not find significant differences in fruit mass. However, in our experiment, it was observed that PC application at sowing provided fruits with greater mass.
The application of soluble salts, such as potassium chloride, facilitates nutrient leaching due to water solubility and high doses applied to the soil (Van Raij, 2011). Potassium nitrate and sulfate are solubilized more gradually in the environment and have other elements, such as sulfur, magnesium, and/or nitrogen, in their composition. They also have a lesser saline effect and are less harmful to plants; however, they are less used since they are more expensive than potassium chloride that is the most commonly used source (Yamada & Roberts, 2005).
Another natural potassium source that can be used in agriculture is Ekosil™ that contains around 8% K20 and 25% Si, and has slow solubility, releasing the potassium gradually into the soil. However, there is no evidence in the literature that the application of Ekosil™ in sowing fertilization could affect the topdressing fertilization requirements or how it affects the quality of the melons.
Kano et al. (2013), studying hybrids of cantaloupes, reported that the most extracted nutrients by the fruits are potassium, nitrogen, and calcium, and this will directly affect the quality of the fruits after harvest. The topdressing application of potassium sulfate and Ekosil™ without sowing fertilization with this natural product resulted in larger fruits since the nutrient was available to the plant during the period of greatest nutritional demand. This provided greater assimilation of photoassimilates due to the increase in the photosynthetic rate and greater water intake due to the lower osmotic potential in the presence of this nutrient.
Van Raij (2011) reported that potassium directly influences fruit characteristics due to the displacement of this nutrient throughout the plant. However, Wade et al. (2004), when applying potassium nitrate to melons, did not observe differences in fruit weight gain, corroborating the results found in our study.
According to Braga et al. (2010), fruits with values of soluble solids above 9.0 °Brix are considered quality fruits with good consumer acceptance. Asao et al. (2013) and Mohammadrezakhani et al. (2016) reported that potassium has a direct influence on the levels of soluble solids as it is a nutrient that is part of the metabolic process of melon fruits together with the ambient temperature that influences the flavor and fruit aroma, characteristics that are decisive at the time of marketing. Castoldi et al. (2008) found that lace melon hybrids with soluble solids ranging between 9.0 and 11.2 °Brix were fully accepted by the final consumers. Lester et al. (2010) observed that the levels of sucrose, glucose, and fructose in melons increased with the supplementary foliar fertilization with potassium.
The levels of organic acids normally decrease with the maturation of the fruits. These acids are also important sources of energy in the respiratory process and are converted or oxidized to sugars and used by the cells (Batista-Silva et al., 2018). A climacteric fruit at harvesting will show greater sugar accumulation and less acidity. This fact was observed in cantaloupes fertilized with topdressing potassium sulfate without sowing fertilization with Ekosil™ that showed low acidity when compared to other treatments. This may have occurred because these potassium sources are slow-releasing, and the fruit absorbs the nutrient in the ideal phase of its reproductive process.
According to Chitarra and Chitarra (2005), the chemical indexes that better demonstrate the maturation point are pH, TA, and SS content. These authors also reported that RA is one of the best methods to evaluate the taste of fruits since the higher the value, the greater the amount of sugars, and the lower the acidity; this enables the perception of sweetness in the fruit that is one of the most relevant attributes for the consumer (Jordan & Seelye, 2009). The results of these reports agree with the SS and TA observed in this study, in which the treatment subjected to topdress-ing fertilization with potassium sulfate without sowing fertilization with Ekosil™ resulted in a greater content of SS and a lower content of TA, obtaining sweeter fruits. The increased potassium fertilization also increases its concentration in the fruits, improving its distribution and resulting in greater neutralization of acids and sweeter fruits (Grangeiro & Cecilio Filho, 2004). Feltrin et al. (2005) observed that the SS/TA ratio in tomatoes was not altered with topdressing fertilization with potassium chloride and potassium sulfate; however, the use of potassium sulfate here resulted in the increase of the ratio.
The potential of hydrogen is based on an index that represents the acidity, neutrality, or alkalinity of any medium. The pH value can indicate the time of fruit ripening and, thus, estimate the precocity. According to Batista-Silva et al. (2018), the acidity of the fruit generally tends to decrease due to the use of organic acids in the fruit's respiratory process that is intense as it grows and matures. For Max et al. (2010), the citric acid that initiates the reactions of the Krebs cycle and other organic acids used as intermediates in biochemical reactions may have their concentrations reduced in the pulp. In this trial, no pH variations were observed for cantaloupes fertilized with different topdressing potassium sources associated or not with sowing fertilization with Ekosil™. Malta et al. (2013) observed a reduction in acidity in guavas treated with the combination of cattle manure and rock powder, which is a desirable characteristic for fresh consumption.
Regarding chlorophyll and carotenoid contents, differences among the treatments were expected since chlorophyll degradation and carotenoid synthesis are processes that involve the differentiation of chloroplasts into chromoplasts that are plastids that accumulate carotenes (Li & Yuan, 2013). This occurs mainly during the ripening process of climacteric fruits. Carotenoids are divided into primary metabolites that are responsible for the survival of the plant, performing an active function in the processes of photosynthesis, respiration, and assimilation of nutrients and secondary metabolites responsible for the stabilization of the plasma membrane (Isah, 2019). In a study conducted by Lester et al. (2010), the beneficial effects of K, via the leaf, occurred due to the increase in the photosynthetic rate, improving the assimilation and translocation of photo-assimilates from leaves to fruits and causing greater activation of the lipoxygenase enzyme and availability of substrate for ascorbic acid and ß-carotene biosynthesis. However, in this trial, we did not observe variations in pigments when various topdressing potassium sources were associated or not with sowing fertilization with Ekosil™. K acts as a cofactor for specific enzymes in the formation of pigments and, therefore, influences the increase in red coloration in the epidermis (Trevisan et al., 2006). However, this was not observed in this study. Medeiros et al. (2014) observed an increase in the content of carotenoids in passion fruit peel when associated with rock powder and bovine biofertilizers.
Conclusions
Under the edaphoclimatic conditions of the Pontal do Triângulo Mineiro region, the use of topdressing fertilization with potassium sulfate without sowing fertilization with Ekosil™ resulted in heavier and sweeter cantaloupes that are crucial factors for greater acceptability of the product by the consumer market. Further studies are needed to evaluate the fertilization with organic and natural products, especially regarding the absorption and assimilation of nutrients by plants and the interference in the quality and productivity of vegetables.