Introduction
More than 60 infectious/contagious diseases known in Colombia share a clinical presentation known as Acute Febrile Syndrome (AFS) 1. AFS refers to sudden onset of fever of less than 7 days, in patients between 5 to 65 years of age, without an apparent infectious source/focus 1. Dengue Fever (DF) is the main cause of AFS in Colombia, ranging from 37 to 43.6% of the cases, followed by Rickettsiosis and Leptospirosis 1. Knowing the differential diagnosis of the main causes of this syndrome is key to any physician who comes upon this scenario. Wise choices of tests may confirm the most common causes, and less probable diagnoses may be ruled out based solely on the clinical examination.
In the first 26 epidemiological weeks of 2019, 1,297,632 cases of DF were reported in Latin American countries with the confirmation of 46.7% of them 2. According to Colombia’s National Institute of Health, the cumulative cases of DF up to the 23rd week of 2020 are 56,923, pointing Risaralda as a territory with alarming behavior for this disease 3. The diagnosis of DF is essentially made through clinical evaluation 4. Case confirmation requires testing serum for detection of IgM antibodies against Dengue, in which flaws have been evidenced due to the request of the test within the first 5 days of fever onset, a sign of inadequate clinical practice which could lead to false negative cases 5.
There is an underestimation of pathogens that are known to be the cause of AFS but are not tested for, therefore underreported 6. Among these are: Leptospirosis, Rickettsiosis, Bartonellosis, Q fever, Hantavirus, Chagas disease, and endemic invasive mycosis 7. Missing these diagnoses could lead to increased morbidity and mortality among this group of patients. A syndromic approximation of patients with AFS is recommended, separating them in categories according to their associated symptoms such as jaundice (e.g. Hepatitis A/B, Malaria, Leptospirosis, and Yellow Fever), hemorrhagic signs (e.g. Dengue), exanthema (e.g. Dengue, Rickettsiosis, Chikungunya, HIV), adenopathy (e.g. Toxoplasmosis) or unspecific febrile syndrome (e.g. Rickettsiosis, Typhoid Fever) (1).
As fever is one of the most common patient complaints in the Emergency Department (ED) (8), the objective of this paper is to determine the current state of clinical practice and to identify alternative diagnoses among those patients with clinical suspicion of DF, who had negative IgM serum testing, presenting to a referral center of Pereira, Colombia.
Methods
Setting
The hospital is in Pereira, Risaralda, Colombia (4°48’53’’N, 75°41’40’’O), at 1411 meters above sea level (MASL) with an annual mean temperature of 22°C, a relative humidity varying between 74% and 79%, and a total area of 702 square kilometers. The estimated population is of 443,554 people according to the National Administrative Department of Statistics (DANE). This institution is a third level care and referral center for people from all the 14 municipalities in Risaralda, as well as some near municipalities of two neighboring departments: Caldas and the north of Valle del Cauca.
Subject of study/Population
Clinical records were obtained for this study from patients who were five years and older that attended the ED of ESE Hospital Universitario San Jorge de Pereira, Colombia between January 2014 and December 2017, and had a Dengue IgM test requested. The first step was the identification of those who tested negative and positive. Inclusion criteria were: patients with a negative IgM test presenting with AFS to the emergency department, who had suspicion of DF. AFS was defined as sudden onset of fever (temperature of 37.6 ºC or higher at admission or during hospitalization) of less than 7 days, in patients who were 5 years or older, without an apparent infectious source/focus. Exclusion criteria used were: first, patients that did not have fever during the period of analysis; and, second, patients with incomplete data for the variables included in the study. Clinical records of patients that tested negative for Dengue IgM test were individually analyzed by all the authors to determine if patients fit the definition of AFS, to apply the remaining exclusion criteria, and to collect all the necessary information to fill out the excel data base. Sample size was not calculated because all patients that presented to the ED during the period of analysis and fulfill the inclusion/ exclusion criteria were included. There was not any direct intervention over clinical practice regarding the attention of the patients included in this study, as well as any practice of laboratory or physical evaluation from the authors.
Variables
The presence of fever was defined as an axillary body temperature registered at admission or during hospitalization of 37.6 ºC or more. Cases were classified according to the syndromic approximation suggested by Cortés JA et al (1). Jaundice was defined as a yellowish discoloration of the skin or mucous membranes with total bilirubin of 1.3 mg/dL or more. Hemorrhagic signs were defined as the presence of any of the following: spontaneous bleeding, petechiae, ecchymoses, hematomas, easy bruising, or a platelet count of less than 120.000/μL.
Exanthema was defined as the presence of red discoloration of the skin or rash recorded during the physical examination. Adenopathy was defined as the presence of enlargement of any lymph nodes registered in the clinical findings. Hepatomegaly was defined as the presence of craniocaudal span of 13 cm or more in ultrasonography or palpation of liver below costal margin. Splenomegaly was defined as the largest dimension of the spleen measuring 13 cm or more in ultrasonography or palpation of the spleen. Anemia was defined as hemoglobin levels of 13 or less for men and 12 or less for women.
Leukocytosis was defined as more than 10,000 WBC/μL. Unspecific febrile syndrome was defined as the presence of fever lacking any group of symptoms that lead towards any specific febrile syndrome (e.g. hemorrhagic, icteric, exanthematic).
Definite diagnoses were stablished based on positivity of the following tests by etiology: Dengue (Dengue IgM), Leptospirosis (Leptospira spp. IgM), Malaria (Malaria Blood Smear), Hepatitis B (Hepatitis B Surface Antigen - HbsAG), Hepatitis C (Antibodies against Hepatitis C), HIV (ELISA), bloodstream infection (Blood culture), Syphilis (VDRL/RPR), Toxoplasmosis (Toxoplasma gondii IgM), and Parvovirus B19 (Blood Marrow Biopsy). Given the recognition in the literature of composite syndromes of AFS, two additional groups were created: exanthematic-hemorrhagic and icterohemorrhagic. These were defined as the simultaneous presence of the manifestations of these syndromes in the same patient. Furthermore, patients were classified in two groups, whether they have a definitive diagnosis or not.
Data collection and source
To describe sociodemographic characteristics, clinical records information was registered in a formulary designed and approved by the research group for that purpose. Variables related to the patients were collected, such as: 1. Age and sex, 2. housing municipality, and 3. clinical manifestations at admission and during the length of stay. The main and only sources of information were the patients’ clinical records.
Statistical methods
Continuous variables were described with medians and interquartile ranges (IQR). Categorical variables were described as proportions. A bivariate analysis was made according to clinical and sociodemographic characteristics among each category. Statistical significance among groups was evaluated using Chi-Square test or Fisher’s exact test for categorical variables and Mann-Whitney U Test for comparing medians. The data were analyzed using Microsoft Excel software and STATA 16.0.
Ethics statement
This descriptive study was approved by the Institutional Research Board of the ESE Hospital Universitario San Jorge from Pereira and the Ethics Committee of the Universidad Tecnológica de Pereira, Colombia. It waived the need for informed consent of the subjects, given that this was a historical review of patient records, and no interventions were done. This was classified as an investigation without risk according to national and international guidelines. The data collection was made following the recommendations of good clinical practice guidelines and the declaration of Helsinki.
Results
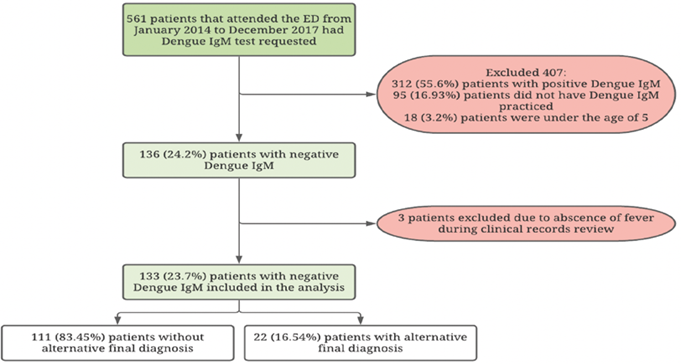
Throughout 2014 until 2017, 561 patients that attended the ED of ESE Hospital Universitario San Jorge had Dengue IgM test requested (Figure 1). DF diagnosis was confirmed in 312 cases (55.6%). 95 patients (16.9%) had Dengue IgM requested but it was not practiced. 154 cases (27.4%) tested negative for Dengue IgM (Table 1). Among these patients, 3 cases (2%) had no fever during observation and 18 patients were under the age of 5, therefore were excluded, leaving 133 (23.7%) patients for analysis. 54.9% (n=61) of analyzed patients were females, the median age was 26 years (IQR 18-47), with 22% coming from rural areas, a median of 4 days of fever at admission (IQR 3-6) and a median of 5 days (IQR 3-7) from fever onset to DF IgM testing. 81 cases (60.9%) had IgM test for DF done before the sixth day of illness.
Table 1: Patient characteristics of unconfirmed dengue cases according to final diagnosis status
| Variables | No Alternative diagnosis | Alternative diagnosis | p |
|---|---|---|---|
| Total (%) n=111 | Total (%) n=22 | ||
| Female | 61 (54.9) | 10 (45,5) | 0.4146 |
| Age (IQR) | 26 (18-47) | 36.5 (22-52) | 0.2297 |
| 5 to 14 y | 15 (13.5) | 3 (13.6) | |
| 15 to 65 y | 87 (78.4) | 17 (77.3) | 1.0008 |
| More than 65 y | 9 (8.1) | 2 (9.1) | |
| Procedence | |||
| Rural | 22 (19.8) | 9 (40.9) | 0.0336 |
| Insurance status | |||
| Subsidiated | 44 (39.6) | 8 (36.6) | |
| Contributors | 51 (45.9) | 8 (36.6) | |
| Indigenous | 0 (0) | 1 (4.5) | 0.2166 |
| Vinculated | 13 (11.7) | 4 (18.2) | |
| Military forces | 3 (2.7) | 1 (4.55) | |
| Days of fever at admission (IQR) | 4 (3-6) | 5.5 (4-8) | 0.0087 |
| Days of fever at Dengue IgM test | 5 (3-7) | 5.5 (4-10) | 0.0667 |
| Headache | 70 (63.1) | 14 (63.6) | 0.9596 |
| Myalgia | 79 (71.2) | 16 (72.3) | 0.8836 |
| Respiratory manifestations | 4 (3.6) | 3 (13.6) | 0.0898 |
| Hepatosplenomegaly | 10 (9.0) | 5 (22.7) | 0.0758 |
| Thrombocytopenia1 | 47 (42.3) | 13 (59.1) | 0.1496 |
| Leukocytosis2 | 14 (12.6) | 7 (31.8) | 0.0488 |
| Anemia3 | 37 (33.3) | 18 (81.8) | <0.0016 |
| Hyperbilirrubinemia4 (n=71) | 5/52 (9.6) | 7/19 (36.8) | 0.012 |
| BUN > 25 mg/dL | 3 (2.7) | 6 (27.3) | <0.0018 |
| Creatinine > 2 mg/dL | 0 (0) | 2 (9.1) | 0.0168 |
| Hospital stay (IQR) | 3 (1-4) | 4 (2-8) | 0.0357 |
| Death as outcome5 | 3 (2.5) | 2 (6.7) | 0.0328 |
Abbreviations: n: sample number, p: probability, IQR: interquartile range, mg: milligrams, dL: deciliter, IgM: immunoglobulin M. 1Platelets < 100.000/μL. 2Leukocites >10.000/μL. 3Hemoblogin < 13 g/dL in males and < 12 g/dL in females. 4Total bilirubin > 1.2 mg/dL. 5Status at discharge. 6Pearson chi2. 7Mann-Whitney. 8Fisher’s exact test. Source: own creation
The three main alternative diagnoses were leptospirosis (7.5%, n=10), malaria (3.8%, n=5) and bacterial bloodstream infection (2.3%, n=3) (Table 2). Only 22 patients (16.54%) without DF confirmation had a final confirmed alternative diagnosis. Of these patients, a statistically significant proportion were from rural origin and coursed with anemia, leukocytosis, hyperbilirrubinemia, icterohemorrhagic and icteric AFS. The three most common AFS presentations were hemorrhagic (34.6%, n=46), exanthematic (21.1%, n=28) and undifferentiated (21.1%, n=28) (Table 3). Myalgia (71.2%, n=79) and headache (63.1%, n=70) were the most common clinical manifestations.
Table 2: Diagnostic test results distribution
| n=133 | Positive Total (%) | Negative Total (%) | Not Done Total (%) |
|---|---|---|---|
| IgM Leptospira | 10 (7.5) | 32 (24.1) | 91 (68.4) |
| Malaria blood smear | 5 (3.8) | 44 (33.1) | 84 (63.2) |
| Blood cultures1 | 3 (2.3) | 10 (7.5) | 120 (90.2) |
| HIV ELISA | 2 (1.5) | 54 (40.6) | 77 (57.9) |
| HBsAg | 0 (0) | 42 (31.6) | 91 (68.4) |
| HCV Ab | 0 (0) | 18 (13.5) | 115 (86.5) |
| VDRL/RPR | 2 (1.5) | 39 (29.3) | 92 (69.2) |
| IgM Toxoplasma gondii | 2 (1.5) | 3 (2.3) | 128 (96.3) |
| BMB for PVB19 | 1 (0.7) | 0 (0) | 132 (99.3) |
Abbreviations: MBS: malaria blood smear, HIV: human immunodeficiency virus, ELISA: Enzyme-Linked Immunosorbent Assay, IgM: immunoglobulin M, HBsAg: hepatitis B surface antigen, HCV: hepatitis C, Ab: antibody, VDRL: Venereal Disease Research Laboratory, RPR: Rapid Plasma Reagin, BMB: Bone Marrow Biopsy. 13 for gram negative bacilli (2 Escherichia coli and 1 Klebsiella pneumoniae). Source: own creation
Table 3: Syndromic presentation of unconfirmed dengue cases according to final diagnosis status1
| Variables | No Alternative diagnosis Total (%) n=111 | Alternative diagnosis Total (%) n=22 | Total (%) n=133 |
|---|---|---|---|
| Undiferentiated | 24 (85.7) | 4 (14.9) | 28 (21.1) |
| Hemorrhagic | 38 (82.6) | 8 (17.4) | 46 (34.6) |
| Exanthematic | 27 (96.4) | 1 (3.6) | 28 (21.1) |
| Exanthematic-hemorrhagic | 15 (88.2) | 2 (11.7) | 17 (12.8) |
| Icterohemorrhagic | 6 (54.5) | 5 (45.5) | 11 (8.3) |
| Icteric | 1 (33.3) | 2 (66.7) | 3 (2.3) |
1Fisher’s exact test p=0.010. Source: own creation
In 84 patients (63.2%) with dengue-like AFS, no tests for malaria (Blood Smear) were performed. HIV testing (ELISA) was only carried in 56 cases (37.08%), IgM for leptospirosis in 42 cases (31.57%), and blood cultures were drawn in only 13 cases (9.77%). Coinfections were detected: 2 (1.3%) patients tested positive for VIH (ELISA test), one of them also had a positive test for Syphilis (Positive VDRL) and the other one for Toxoplasma gondii (IgM test); and 1 patient (0.66%, n=1) tested positive for both malaria (Malaria Blood Smear) and leptospirosis (IgM test).
Patients with a definitive diagnosis had a statistically significant longer hospital stay (3 (IQR 1-4) vs 4 (IQR 2-8) days, p=0.035) and greater mortality (3/111 (2.5%) vs 2/22 (6.7%), p=0.032). On the other hand, among patients without a definitive diagnosis and an undifferentiated or icterohemorrhagic presentation of AFS, blood cultures and Hepatitis C antibodies were the most used tests. In general, the use of malaria (29.7%, n=33), HIV (42.3%, n=47) and leptospirosis (20.7%, n=23) testing was low in all subgroups of AFS without a definitive diagnosis (Table 4), and these patients did not have any specific clinical presentation favoring its utilization.
Table 4: Tests ordered in the patients without alternative diagnosis according to febrile syndrome
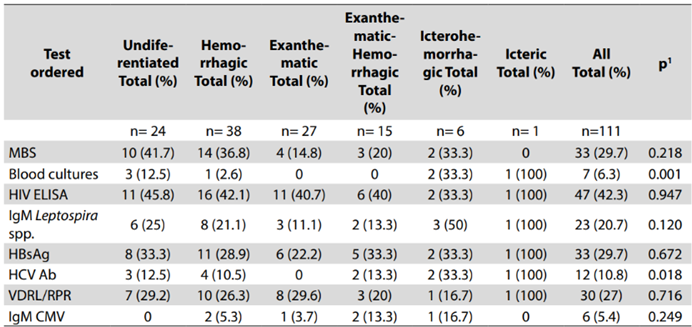
Abbreviations: MBS: malaria blood smear, HIV: human immunodeficiency virus, ELISA: Enzyme-Linked Immunosorbent Assay, IgM: immunoglobulin M, HBsAg: hepatitis B surface antigen, HCV: hepatitis C, Ab: antibody, VDRL: Venereal Disease Research Laboratory, RPR: Rapid Plasma Reagin. 1Fisher’s exact test. Source: own creation
Discussion
The findings from this study highlight the need of reinforcing clinical practice concerning the approach to patients presenting with AFS to the emergency department. The most common etiologies for AFS were, in a considerable proportion, not properly addressed according to the number of tests done for these (malaria, leptospirosis, rickettsiosis, etc.). The use of diagnostic test in patients not meeting clinical case definitions was common. Regardless, our findings suggest that the three main detected causes of Dengue-like AFS were: leptospirosis, malaria, and bloodstream infection.
Risaralda is a department, in average, under 1516 MASL, what makes it the perfect place for any cause of AFS to occur. 21.8% (n=74,596) of the population in Risaralda lives in rural areas 9, which have sanitary deficits such as inappropriate waste disposal and water drains; cultural/educational factors that condition poor hygiene, inappropriate living conditions, unsafe sex, and unhealthy food source that could also play a determinant role in the incidence of AFS 10.
These conditions are relevant since a significant proportion of patients with alternative diagnosis were from rural origin, a population characterized for having the highest incidence of malaria and leptospirosis in Risaralda, the two main alternative diagnoses in this study. Consistently, 22% of the analyzed patients were from rural origin. The association of anemia, leukocytosis, hyperbilirubinemia, and acute kidney injury with final alternative diagnosis can be explained by their occurrence in the clinical course of any of the three main alternative causes of Dengue-like AFS identified in our study. Regarding alternative diagnosis to Dengue, the underutilization of tests to diagnose leptospirosis and malaria, which are among the three main etiologies of AFS and AUFI in Colombia, is of concern 11,12. Exanthematic, hemorrhagic, and undifferentiated AFS favored having a final diagnosis at discharge, which is related to our findings of leptospirosis and malaria being the two main alternative diagnoses, as both can cause any of these three clinical profiles. We were not able to rule out DF in 60.9% of the cases, as IgM test was drawn within the first 5 days of fever, leading to a large potential of undiagnosed DF cases 13,14. In a referral center of a South American country, over 80% of cases with DF-like AFS were ruled out without an etiological diagnosis 2.
Despite being an endemic area for malaria and having a high frequency of leptospirosis, rickettsiosis and HIV, the active search of these entities is not taken into account during the study of undifferentiated and icterohemorrhagic AFS 2. Interestingly, among our patients without a definite diagnosis, whose main syndromic presentation was icterohemorrhagic and undifferentiated fever, there were no significant number of tests to identify leptospirosis or rickettsiosis, the latter due to no availability of Indirect Immunofluorescence (IIF) test. Overall, testing for leptospirosis was low, even in patients with alternative diagnoses.
This reinforces the concept of leptospirosis as a neglected disease. Faccini-Martínez et al. reported comparable proportions of patients who ended up having no confirmed alternative diagnoses (86.6% vs 83.4%) 15, where our findings of bloodstream and HIV infection become relevant as they could explain the clinical profile of some patients presenting with AFS.
Other studies have approached our objective from a different point of view. Arroyave et al. 12 analyzed 220 cases with non-malaria AFS, finding DF (37.3%) as the main cause followed by Leptospirosis (14.1%), and Rickettsiosis (2.7%). Leptospirosis represented 8.2% of cases with alternative diagnosis among patients that did not have Dengue, which is comparable to our rate of diagnosis (7.5%). The difference between our study and Arroyave’s lies in that they practiced tests for leptospirosis, dengue, rickettsiosis, arenavirus, and hantavirus in all the patients included in their study. 68.4% of our patients did not have tests performed for Leptospirosis and Arroyave’s sample is small as ours, therefore both of our rates of isolation are not a true reflection of what we could encounter in clinical practice.
Only one study in Brazil during 2008 evaluated other causes of AFS in patients with a negative IgM for dengue, finding a definitive diagnosis in 27.1% of the cases. The results were limited because investigators restricted testing to Dengue, Rubella, Leptospirosis and Hantavirus, showing an 11.8% seroconversion for dengue and leptospirosis as the main cause (13.9%) 16.
Two other studies were conducted in Colombia, one on non-malarial AFS 12 and one on AUFI 11, those studies have shown that there is an increase in the rate of final diagnosis (between 42.7% to 69% of cases) if routine testing for hantavirus, leptospirosis, dengue, malaria and rickettsiosis are systematically done. Also, these studies showed that, although there is a widespread idea that certain manifestations could help to clinically differentiate patients with these infections, it is frequent to find coinfections and there are no statistically significant differences between manifestations among these etiologies. Clinical case definition for dengue, leptospirosis, and malaria in Colombian guidelines overlap in several manifestations. Hence, the clinical picture could lack specificity and can be even misleading, which can explain why we had 3 patients with coinfections as they were tested to narrow down diagnostic possibilities, findings also reported in other studies 12.
Since this was a retrospective study of clinical records, it has several limitations. First, information collected was limited to what was written by the attending physician. Therefore, clinical data necessary to specify the syndromic profile and the remaining variables were not widely available. Second, there was a scarce availability of the laboratory tests needed to rule out Rickettsia spp. (IIF), typhoid fever (PCR), Leptospira spp (PCR and microagglutination) and arboviral infections besides DF. Third, the high proportion of DF IgM tests taken before the 6th day of fever could have led to a higher proportion of negative DF cases because of misdiagnosis. Fourth, this study was conducted on a small sample over a short period of time. Fifth, laboratory tests practiced in the differential diagnosis lacked complementary tests (e.g Hepatitis B virus anti core IgM and IgG), context (e.g Toxoplasma gondii IgM positivity lasting beyond 1 year), appropriateness (e.g Parvovirus B19 IgM serology instead of Bone Marrow Aspiration) or were not available (e.g Viral panels for Zika, Chikungunya, and IIF
for Rickettsiosis). Sixth, with the available data, it was not possible to know the exact prevalence of AFS that is caused by leptospirosis, typhoid fever, rickettsiosis, malaria, or acute retroviral syndrome. Hence, it reflects rates of isolation.
Overall, we found that there is an increasing need of reinforcement of clinical practice when approaching to patients with AFS. Differential diagnosis should take in account local epidemiology and clinical presentations that favor specific laboratory testing, two main items we intended to contribute to with our results. Further research is needed to determine the impact of training the ED personnel in the recognition of AFS with respect to improvement in quality of attention, and rates of diagnosis. Results from these types of investigations might improve the capacity and availability of tests for neglected and underreported causes like rickettsiosis and leptospirosis. Once this is accomplished, the real impact of these zoonotic AFS in Colombia could be known. Local common causes of AFS should be recognized to ensue provisional adequate treatment and targeted laboratory evaluation.