INTRODUCTION
Siphonophores are pelagic, complex colonial and polymorphic hydrozoans currently comprising 190 valid species (Schuchert, 2022). They mostly inhabit oceanic waters; few neritic representatives have been recorded and only the Family Rhodaliidae is epibenthic. Some small species are restricted to epipelagic zones, others to mesopelagic and bathypelagic areas, making them possible indicators of water masses (Pugh, 1999; Palma and Silva, 2006; Dunn et al., 2005a; Mapstone, 2014).
Siphonophores have great ecological importance and make a significant contribution (~25% of the total pelagic biomass) to the trophic links in the deep sea (Robison, 2004; Mapstone, 2014); their abundance, along with that of other planktonic cnidarians, can affect copepod populations through predation, thus decreasing the amount of food available for economically important fish species (Pugh, 1999; Pagès et al., 2001).
Siphonophore bodies have three parts: the pneumatophore (float), the nectosome which bears the propulsion nectophores and the siphosome, which bears the gonophores, gastrozooids and palpons; the order is subdivided into three sub - orders, Cystonecta, Physonectae and Calycophorae, depending on the presence or absence of some of these parts (Dunn et al., 2005a; Mapstone, 2014) (Fig. 1). They are difficult to collect; their bodies are extremely fragile and many are found only in the deep sea (Dunn et al., 2005b; Haddock et al., 2005). During a tow zooplankton collection, the net breaks the long - stemmed bodies of siphonophores into pieces, so the identification of this group is based mostly on body parts of the polygastric colony (nectophores, bracts or pneumatophores) or the free - living sexual stage (eudoxids with gonophores). However, there have been cases of misunderstanding due to the identification of each body part as a different organism.
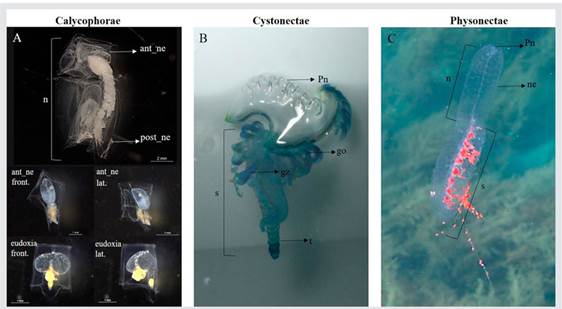
Figure 1 Photographs of representatives of the three sub - orders of siphonophores taken in situ and in the laboratory. (A) Calycophorae, Abylopsis tetragona, two nectophores locked together in the polygastric stage (Photo: Gil, L. 2022 - Pacific Ocean); anterior nectophore frontal and lateral view and eudoxia phase frontal and lateral view (Photos: Dorado - Roncancio. 2014 and 2015 - Caribbean Sea). (B) Cystonecta, Physalia physalis (Photo: Londoño, E. 2010 - Pacific Ocean); (C) Physonectae, Agalma okenii (Photo: Andres Rojas, 2019 - Caribbean Sea). Labels: go = gonophore; gz = gastrozooid; ne = nectophore (swimming bell); ant_ne = anterior nectophore; post_ne = posterior nectophore; pn - pneumatophore (float); t - tentacle.
Most siphonophores have cosmopolitan geographical distributions, however, there are species known only from a single locality and others that are restricted to specific latitudinal ranges (Alvariño, 1971; Mapstone, 2014). The study of the siphonophores is very limited in Colombia, because there are few researchers working with this group. Records of siphonophores are scattered in unpublished literature and in some publications focused on coastal planktonic communities (specifically zooplankton); they are overlooked or underestimated, identified only to order (Cepeda, 2007; Giraldo and Gutierrez, 2007; Martinez et al., 2007; Giraldo et al., 2014; Contreras - Vega et al., 2021). Therefore, little is known about their taxonomy, horizontal and vertical distribution, feeding habits and abundance.
As a contribution to the national inventory of marine diversity in Colombia, the present list of siphonophore species recorded in the Colombian Caribbean Sea and Pacific Ocean is based on secondary information and from the oceanographic campaigns carried out in the framework of agreements between Marine and Coastal Research Institute “Jose Benito Vives de Andreis” (INVEMAR) and the National Hydrocarbons Agency (ANH).
STUDY AREA
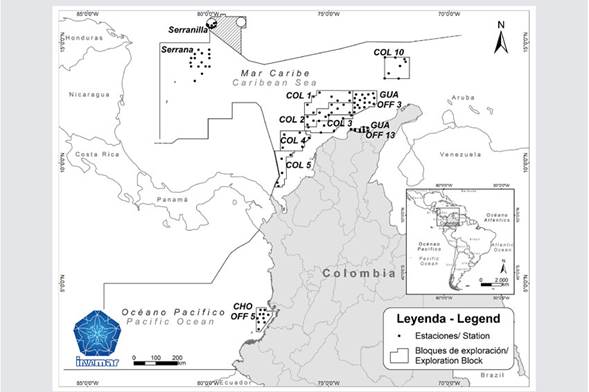
Colombia is the only country in South America with coasts on the Pacific Ocean and Caribbean sea; about 44.86% of its national territory is composed of maritime and insular areas (Fig. 2). The Colombian Caribbean Sea comprises the territorial oceanic waters beyond the continental shelf and reaches the Exclusive Economic Zone, where depths range from 130 m to 4200 m; it is bordered by Panama, Costa Rica, Nicaragua, Honduras, Jamaica, Haiti, Dominican Republic and Venezuela, including San Andrés and Providencia Islands and the Key Islands located in the western Caribbean Sea (Albuquerque, Cayo de Este, Roncador, Serrana, Quitasueño, Serranilla and Bajo Nuevo). The Colombian Pacific includes the waters from the coastline between the maritime borders of Costa Rica, Panama and Ecuador; to the outer limit of the Exclusive Economic Zone (EEZ) including the islands of Malpelo, Gorgona and Gorgonilla, with depths reaching 4000m.
METHODS
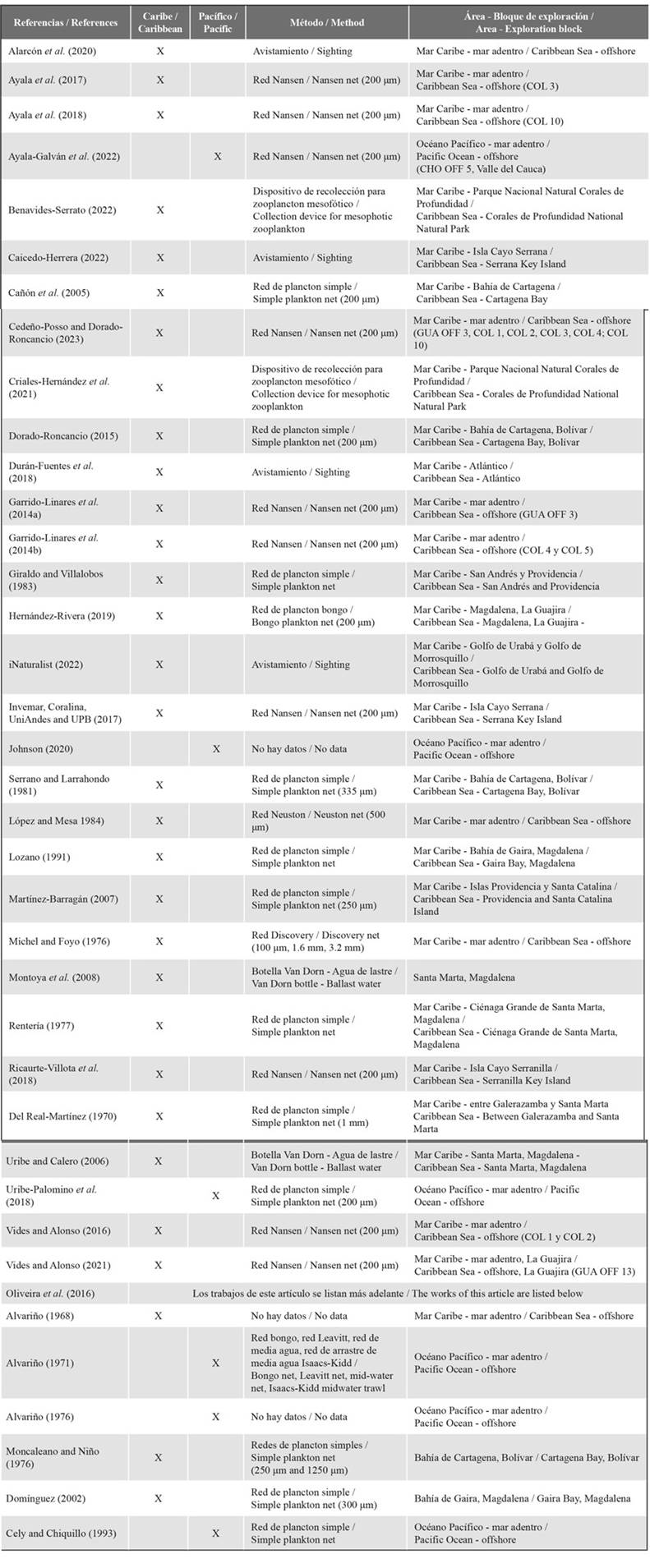
A review was carried out of the records of genera and species of siphonophores in the Colombian Caribbean Sea and eastern Pacific Ocean. Previous records of Colombian siphonophores, including grey literature, are already inventoried in the work of Oliveira et al., 2016, who included data from the Caribbean Sea (Alvariño, 1968; Moncaleano and Niño, 1976; Dominguez, 2002); metadata from Dominguez (2002) can also be accessed in the Ocean Biodiversity Information System (OBIS) and Global Biodiversity Information Facility (GBIF) (Montoya - Cadavid and Bohórquez. 2022), and from the Pacific Ocean (Alvariño, 1971; Alvariño, 1976; Cely and Chiquillo, 1993) (Table 1).
The new data from the Caribbean Sea that contribute to the list come from: Michel and Foyo, 1976; Renteria, 1977; Giraldo and Villalobos, 1983; López and Mesa, 1984; Lozano, 1991; Cañón et al., 2005; Montoya et al., 2008; Medellín - Mora and Martínez - Ramírez, 2010; Durán - Fuentes et al., 2018; Alarcón et al., 2020; Cedeño - Posso and Dorado - Roncancio, 2023. Considering that studies are scarce, records from seven undergraduate and master’s theses were included as possible observations of the species in the area, however, as they are records that do not have a voucher in a museum to be confirmed, they should be used with caution. (Del Real - Martínez, 1970; Serrano and Larrahondo, 1981; Uribe and Calero, 2006; Martínez - Barragán, 2007; Barón, 2007; Dorado - Roncancio, 2015; Hernández - Rivera, 2019). Data for the eastern Pacific Ocean are from Uribe - Palomino et al., 2018. in which samples were collected at 35 oceanographic and biological stations, located within 77 - 84°W and 2 - 6°N, as part of the Regional Study of the El Niño Phenomenon (ERFEN - Colombia) between 2001 and 2004 (Table 1). The list is complemented with the occurrences of siphonophores found in OBIS and GBIF. The following datasets are available for the Caribbean: Criales - Hernández et al., (2021), Benavides - Serrato (2022), Caicedo - Herrera (2022), iNaturalist, 2022 and for the Colombian Pacific: Johnson (2020).
Also included in the list are the results of 11 research projects carried out by INVEMAR between the years 2013 and 2022. Nine projects were in the framework of the agreements between INVEMAR and the National Hydrocarbons Agency (ANH) in seven hydrocarbon exploration areas: GUA OFF 3 (2013), COL 4 (2014), COL 5 (2014), COL 2 (2015), COL 1 (2016), COL 3 (2017), COL 10 (2018), GUA OFF 13 (2020) and CHO OFF 5 (2022) (Fig.2) (Garrido - Linares et al., 2014a; Garrido - Linares et al., 2014b; Vides and Alonso, 2016; Ayala et al., 2017; Ayala et al., 2018; Vides and Alonso, 2021; Ayala - Galván et al., 2022). The other two projects with siphonophore data were done in the Seaflower Biosphere Reserve, as part of the Seaflower Scientific Expeditions 2016 and 2017 (Serrana Key Island and Serranilla Key Island, respectively) (Fig. 2; Table 1), organized by the Comisión Colombiana del Océano (Invemar, Coralina, UniAndes and UPB, 2017; Ricaurte - Villota et al., 2018). These projects performed vertical zooplankton deep - water trawls (Nansen net of 200 μm, 0.6 m mouth diameter and Hydrobios flowmeter) at four different depth ranges (0 - 60 m, 70 - 140 m, 170 - 340 m, 540 - 1000 m) using a GeneralOceanics double trip mechanism. Samples were narcotized in situ with 10% magnesium chloride, fixed with formaldehyde and neutralized with sodium tetraborate (borax), leaving the solution at a concentration of 5%. The siphonophores were separated, identified (Totton, 1965; Pugh, 1999; Licandro et al., 2017) and deposited in the Museo de Historia Natural Marina de Colombia (MHNMC) of INVEMAR; metadata are available in the Marine Biodiversity Information System (SiBM - OBIS Colombia), OBIS and GBIF (Dorado - Roncancio, 2017; Diaz - Hernandez et al., 2021). 75% of the records were from epipelagic samples; only 25% in the last 8 years have been based on epi - and mesopelagic samples of zooplankton. This information was used to generate a comparative taxonomic list with the number of species present in the Colombian seas, their spatial distribution and their respective references.
RESULTS
There are 63 records of species of siphonophores in Colombian marine waters synthesized in Table 2; 50 are identified as unique species and 13 are morphotypes for which the identification is assigned as “sp.”. They include all previous records together with those from the research projects reported here. The names used in this table are those currently recognized as valid (Schuchert, 2022). The siphonophores are arranged in the three main suborders, 9 families and 26 genera. Suborder Calycophorae presented the highest number of species (48) (Fig. 3); the family Diphyidae had the greatest richness of genera (seven) and species (30). Suborder Physonectae has 11 species; the family Agalmatidae showed the greatest richness of genera (four) and species (eight). Finally, Suborder Cystonectae has four species; the family Rhizophysidae had the greatest richness of genera (three) (Table 2).
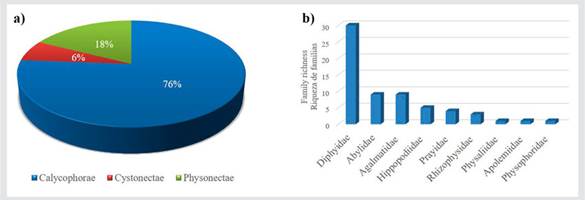
Figure 3 a) Percentage of species of the suborders of siphonophores; b) Siphonophore family richness of the suborder Calycophorae.
The following are some taxonomic annotations on the species reported in Table 2. Species reported for the Caribbean Sea as morphotype “sp.”, like Bassia sp. and Enneagonum sp. reported by Del Real - Martinez (1970) and Serrano and Larrahondo (1981) were listed as Bassia bassensis and Enneagonum hyalinum, because they are monotypic genera. It is most probable that Chelophyes sp. reported by Del Real - Martinez (1970) is Chelophyes appendiculata; the species C. contorta reported for the Pacific Ocean needs confirmation, because it is probably a misidentification of C. appendiculata (Oliveira et al., 2016). There is another doubtful record reported by Domínguez (2002), Diphyes chamissonis; it is subject to further confirmation (Oliveira et al., 2016). Five morphotype ‘species’ from Suborder Calycophorae, recorded only in unpublished manuscripts, require further identification efforts to reach species level (Abylopsis sp., Diphyes sp., Eudoxoides sp, Lensia sp., Muggiaea sp.). The physonect Halistemma sp. could be H. rubrum or H. striata according to the records, but only the bracts were collected, so it needs more revision.
Table 2 Taxonomic list of the species of siphonophores (Cnidaria: Hydrozoa) reported for Colombian marine waters with their respective references. C: Caribbean Sea; P: Eastern Pacific Ocean.
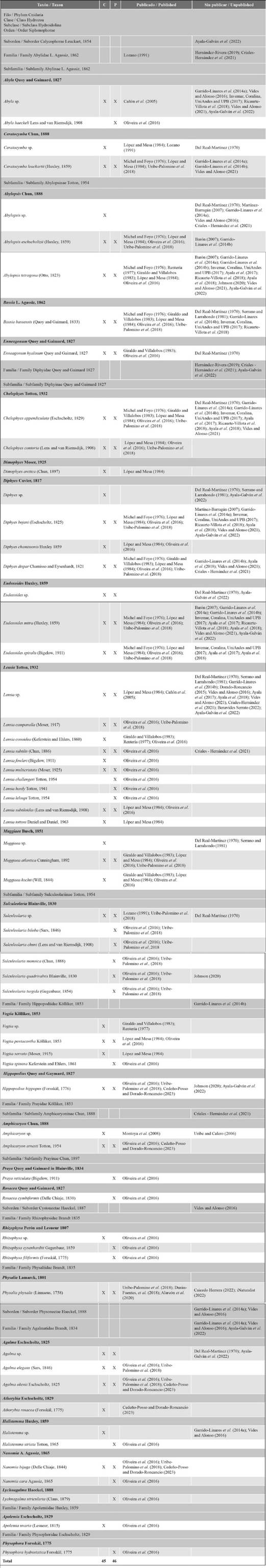
Although some of the records found were housed in public access databases such as OBIS and GBIF, it is necessary to use this information with discretion since they are not identified to the lowest level (Criales - Hernandez et al., 2021; Benavides - Serrato, 2022); some cases, such as the dataset published by American Museum of Natural History (Johnson, 2020) has issues like: “Country derived from coordinates” or “Occurrence status inferred from individual count”. It is valuable information that requires data cleaning and may also need to be peer reviewed and published like a data paper.
Species richness varied between the Caribbean Sea and the eastern Pacific Ocean, even though they share 24 species. The Caribbean Sea has 45 species of siphonophores; nine have only been collected there, while the Pacific Ocean has 46 species and 18 species have only been collected there. The vertical distribution of the species shows that 47 are epipelagic, 11 are epi - mesopelagic and four are epi - bathypelagic (Diphyes bojani, Vogtia serrata, Agalma okenii and Lychnagalma utricularia), and Physalia physalis that are pleustonic.
DISCUSSION
The Colombian siphonophores are mostly (66%) neritic and oceanic species, widely distributed in tropical and subtropical waters (Pugh and Gasca, 2009; Oliveira et al., 2016). Three suborders are present: Calycophorae, Cystonectae and Physonectae. Calycophores are the most common and frequent; they are easily recognizable by their body (only nectosoma and syphosome, without pneumatophores) (Fig. 2). Seven families are currently recognized: Abylidae, Clausophyidae, Diphyidae, Hippopodiidae, Prayidae, Sphaeronectidae and Tottonophyidae) (Schuchert, 2022). Only four of these families have been recorded in Colombia: Abylidae, Diphyidae, Hippopodiidae and Prayidae. Some of the records, especially in the unpublished literature, report them only to family or genera such as Abylopsis sp., and Diphyidae family: Diphyes sp., Eudoxoides sp., Lensia sp. and Muggiaea sp., genera commonly found near the surface (Totton, 1965). The genus Abylopsis has only two recognized species; Abylopsis tetragona, one of the abundant and most common siphonophores, and A. eschscholtzi (Schuchert, 2022). Examining some diagnostic features of each species in detail, such as the lateral radial canals and length of the posterior nectophore in a complete specimen of the Abylopsis polygastric phase (Tottom, 1965), can help to provide reliable identification for new occurrences in Colombia. Similar identification efforts should be carried out for the genera Diphyes (four spp.), Eudoxoides (two spp.), and Muggiaea (four spp.). The case of Lensia is more complex, because to date 26 species have been recognized (Schuchert, 2022) and 20 species are found in South America (Oliveira et al., 2016), therefore a more detailed study of this group is needed. The number of occurrences may increase, because to date only 10 species of Lensia have been recorded for Colombia.
Cystonectes differ from the other two suborders because they do not possess a nectosome (Fig. 2). Currently two families (Physaliidae and Rhizophysidae) and 5 species are recognized (Physalia physalis, Bathyphysa conifer, Bathyphysa sibogae, Rhizophysa eysenhardtii and Rhizophysa filiformis) (Pugh, 2019; Schuchert, 2022). Three of them are recorded for Colombia; Physalia physalis, also known as the Portuguese Man o’ War, is perhaps the most common. It is easily identified by its purple pneumatophore, sail - shaped and maximum 30 cm height, which keeps it floating and drifting. It is frequent during the first months of the year, with a greater number of sightings between February and April, when they are dragged towards the Colombian Caribbean coast by the action of the trade winds and the surface currents.
Physonectes have a pneumatophore, nectosoma and syphosome (Fig. 2). Currently ten families are recognized (Agalmatidae, Apolemiidae, Cordagalmatidae, Erennidae, Forskaliidae, Physophoridae, Pyrostephidae, Resomiidae, Rhodaliidae and Stephanomiidae) (Schuchert, 2022), only three reported for Colombian waters: Agalmatidae, Apolemiidae and Physophoridae.
Colombian Caribbean Sea. The total number of species reported here (45) is less than the number reported for the Western Caribbean (56 species); almost half of the species known presently for the Gulf of Mexico (82 species). The difference is mostly due to the absence of Colombian samples for some families like Erennidae, Forskaliidae, Pyrostephidae, Clausophyidae and Sphaeronectidae (Gasca, 2009; Pugh and Gasca, 2009).
Some species reported for the Colombian Pacific Ocean could also be found in the Colombian Caribbean Sea, due to their spatial distribution in the Caribbean and Atlantic Ocean (Gasca, 2002; Gasca, 2009; Pugh and Gasca, 2009; Varela, 2012; Oliveira et al., 2016) i.e. Abyla haeckeli, Lensia leloupi, Lychnagalma utricularia, Sulculeolaria biloba, S. chuni, S. monoica, S. quadrivalvis, S. turgida, Rhizophysa eysenhardtii, R. filiformis and Physophora hydrostatica.
Colombian Pacific Ocean. The total number of species reported (46 species) is also low compared to records in the Pacific waters of Ecuador, Mexico and Costa Rica (73 species). (Gasca and Suárez, 1992; Gasca, 2002, Andrade, 2012; Andrade, 2014), mostly due to the absence of Colombian samples from some families like Erennidae, Pyrostephidae, Clausophyidae and Sphaeronectidae, and also some genera, Epibulia, Frillagalma, Gilia, Melophysa and Nectopyramis (Gasca, 2009, Pugh and Gasca, 2009).
Future studies in the Pacific Colombian Ocean could find species reported for the Colombian Caribbean, such as Dimophyes arctica, Lensia conoidea, L. fowleri, Muggiaea kochi and Vogtia serrata previously reported for Pacific waters of Mexico and Costa Rica (Gasca and Suárez, 1992; Gasca, 2002; Fernández - Álamo and Ramírez - Arriaga, 2020), from Ecuador (Andrade, 2012; Andrade, 2014; Andrade, 2019; Castillo et al., 2019) and from Peru to Chile (Oliveira et al., 2016). The case of Apolemia uvaria is interesting because it is only species reported for the Atlantic Ocean (Oliveira et al., 2016), but may also occur in the Pacific, because Gasca (2002) reported it for Mexican Pacific waters. In conclusion, the species that could be exclusive to the Colombian Pacific Ocean could be Praya reticulata, Rosacea cymbiformis, and Nanomia cara, although they are subject to confirmation (Purcell, 1984; Gasca and Suárez, 1992; Gasca, 2002; Gasca, 2009; Oliveira et al., 2016).
CONCLUSIONS
From the review of published and unpublished manuscripts, a list of 63 species of siphonophores in Colombia is consolidated, 45 in the Caribbean Sea and 46 in eastern Pacific Ocean. The main difference in species numbers in the Colombian Caribbean Sea and Pacific Ocean, compared to other regions nearby, is due to the traditional methods for zooplankton collection. The use of simple conical or bongo zooplankton nets allows collecting some siphonophores in Colombia, but a lot of species, mostly of the suborders Cystonectae and Physonectae, are very long organisms (Gasca, 2002) that can be collected better using other kinds of nets such as mesopelagic Tucker, Mocness or CalCOFI nets (Gasca, 2009). 76% of the species listed for Colombia are epipelagic, so it is possible to collect more deep - sea species with these other nets or with the use of submersibles (Dunn et al., 2005a; Mapstone, 2014), through which new species have been described in recent years (Pugh, 1998; Pugh, 2005; Dunn et al., 2005b; Haddock et al., 2005; Siebert et al.,2013).
The number of species recorded in Colombian marine waters could increase when samples from meso - and bathypelagic waters are available and when the siphonophores collected with the ichthyoplankton nets (500 μm) begin to be analysed. This siphonophore checklist is important because it integrates all the studies done in Colombia and contributes to the country’s biodiversity catalogue. Future studies should review narcotized specimens in situ to produce a better checklist, and focus on their seasonal and spatial distribution, especially their vertical distribution in the water column, because many of them are indicators of water masses











 text in
text in