Introduction
Invasive Candidiasis (IC) is the most common fungal disease among hospitalized patients worldwide, and candidemia is the most common clinical manifestation1,2. Candida Spp. is an important cause of bloodstream infections (BSI)4-7. Patients at risk of IC include seriously ill patients admitted to Intensive Care Units (ICU), neutropenic patients with cancer, patients who have undergone surgical procedures and premature neonates3-5. Recently, the annual incidence of candidemia alone was calculated to be 12.8 in 100,000 inhabitants, i.e. approximately 6,296 cases per year4-7.
In Colombia, there are different therapeutic alternatives for the management of IC/candidemia, but no national guidelines are available, and there also was little information available about the local epidemiological profile and clinicaldiagnostic approach-associated costs. The following are recommendations for diagnosis, management and follow-up of IC/candidemia in adult and pediatric patients in hospital setting, hemato-oncological units, and patients in the ICU, including those in the Neonatal Intensive Care Unit (NICU). The process of developing these recommendations included a systematic approach for rating the quality of evidence and the strength of each recommendation (Table 1)8,9and a detailed description of methods, background and evidence summaries supporting each recommendation are also included. Even though candidiasis infection in mucous membranes (including oropharynx, esophagus and genital tract) is not considered a typical Invasive Fungal Disease (IFD), this is included in these recommendations. This consensus was reviewed and endorsed by the Colombian Association of Infectiology (ACIN) and is not intended to replace the clinical approach to the management of patients on an individual basis, but to serve as a guide to the diagnosis and treatment of Candidiasis.
Diagnosis of invasive candidiasis (IC)
I. What is the usefulness of taking blood cultures when IC/candidemia is suspected?
Recommendation
Blood cultures and other sterile organic specimen cultures are considered the cornerstone of diagnosing IC/candidemia (strong recommendation, high-quality evidence)10.
In patients with suspected Candida Spp. IFD the diagnostic performance of a blood culture may be maximized by growing additional subcultures from blood culture bottles, regardless of whether inoculated bottles are positive or not (weak recommendation, moderate-quality evidence)11-13.
II. What is the recommendation about collecting blood cultures and how many blood cultures should be collected in patients with suspected IC/candidemia?
Recommendation
3. The consensus panel recommends collecting blood cultures once a day when an infectious process is suspected (strong recommendation, high-quality evidence)13,14.
4. Any factor that affecting the sensitivity such as blood volume, number of bottles, detection time, inoculum size, type of selected bottle and used culture medium should be considered in order to improve the diagnostic performance of blood cultures (strong recommendation, high-quality evidence) 13,14.
5. The consensus panel considers that conventional blood culture bottles and automated continuous monitoring systems are adequate for the diagnosis of candidemia/ IC. Blood culture bottles with fungus selective medium can optimize the recovery of yeasts (strong recommendation, moderate-quality evidence)14,15.
Do the differences in time-to-recovery of causative agent depend on the identified species?
Recommendation
6. The consensus panel considers that the time to detection and positivity of a blood culture may be affected by the Candida species isolated (strong recommendation, moderate-quality evidence)12,13.
III. Are control blood cultures necessary in patients diagnosed with candidemia/IC?
Recommendation
7. The consensus panel considers that control blood cultures are necessary in patients diagnosed with candidemia/IC (strong recommendation, high-quality evidence.
How many time should elapse between the first positive blood culture and control blood cultures?
Recommendation
8. The consensus panel considers that control blood cultures should be collected every 24--48 hours after the first positive blood culture (strong recommendation, moderate-quality evidence).
If control blood cultures are positive, when should be the next blood culture collected?
Recommendation
9. The consensus panel considers that if control blood cultures are positive the next blood cultures should be collected every 24-48 hours (strong recommendation, moderate-quality evidence).
How many sets of negative blood cultures are required to determine that candidemia/IC has been cleared?
Recommendation
10. The consensus panel considers that repeated sets of blood cultures should be collected, until the mycological clearance of candidemia/IC, with two consecutive negative sets (separated by 48 hours) and patient clinical improvement are documented (strong recommendation, moderate-quality evidence).
11. The consensus panel considers that an optimal detection of candidemia/IC is achieved when ≥ 3 sets of blood cultures are performed with minimum 20 minutes in between samples (strong recommendation, moderate-quality evidence).
IV. What is the usefulness of using predictive indexes (risk score) for initiation of an early antifungal therapy in patients with suspected IC?
Recommendation
12. The consensus panel considers that scores/predictive rules permit the stratification and selection of high risk IC patients who could benefit from early antifungal therapy (strong recommendation, moderate-quality evidence) (Table 3)16-18.
Diagnosis of candidemia
V. How is the conventional diagnosis of a candidemia/IC performed?
Recommendation
13. The consensus panel considers that direct microscopic examination by different preparations and staining, is a quick cost-effective method for initial identification of recognized yeast-like species morphologically such as Candida Spp. (strong recommendation, high-quality evidence)10.
14. Histopathology is a fundamental tool for diagnosis and identification of pathogenic yeasts from tissue samples (strong recommendation, high-quality evidence)15,19,20.
15. The consensus panel considers that the etiological agent isolation by a mycological culture is a critical step in the identification of Candida species causing an IFD (strong recommendation, high-quality evidence)15, >,19,20.
16. Different automated systems provide a reliable method for identification of yeast-like fungi, which along with an analysis software and an advanced expert system, increase the rapidity to obtain mycological results (strong recommendation, high-quality evidence)15,19.
17. The consensus panel considers that the availability and use of different conventional diagnostic methods for detection and identification of yeasts depend on the clinical setting (strong recommendation, high-quality evidence)20,21.
Does the antifungal therapy of choice depend on the identified Candida species?
Recommendation
18. The consensus panel considers that rapid identification of involved yeast-like species and performing antifungal susceptibility tests (AFSTs) are necessary for all IFD clinical isolates (strong recommendation, high-quality evidence)11,22.
19. Candida Spp. antifungal susceptibility profile is closely related to the species; therefore, in most of the cases, identification of the species provides useful and sufficient information for the appropriate choice of a targeted antifungal therapy (strong recommendation, moderate-quality evidence) (Table 4)20,23.
Are the identified species of Candida and the time to initiation of antifungal therapy related with the prognosis of candidemia/IC?
Recommendation
20. The consensus panel considers that patients with a Candida Spp. isolate in blood, regardless of whether the sample was obtained through a catheter or by venipuncture, should receive targeted antifungal therapy (strong recommendation, high-quality evidence) 21.
21. The consensus panel considers that early initiation of antifungal therapy is a key factor associated with a good prognosis of candidemia/IC (strong recommendation, high-quality evidence) 12,14.
22. The consensus panel considers that delay in initiation of the is associated with poor clinical course, higher incidence of breakthrough fungemia and higher mortality rates (strong recommendation, high-quality evidence)13,24.
23. C. krusei, C. tropicalis, C. glabrata or C. auris candidemia/IC have been associated with high mortality rates and C. parapsilosis candidemia/IC has been associated with reduced pathogenicity (strong recommendation, moderate-quality evidence)13.
VI. What is the diagnostic value of in vitro AFSTs?
Recommendation
24. Correct identification of the Candida species is predictive of its likely antifungal susceptibility (or resistance) (strong recommendation, moderate-quality evidence) (Table 4)11,25,26.
25. The consensus panel considers that AFST results should be timely (available in about 3 days) to be clinically useful (strong recommendation, high-quality evidence)23.
26. It should be kept in mind that therapy failure is not necessarily secondary to the antifungal agent-of-choice administration (strong recommendation, high-quality evidence)27.
When are AFSTs recommended? Which commercial methods are recommended?
Recommendation
27. The consensus panel considers that AFSTs provide a base for the choice of an appropriate antifungal therapy for patients on an individual basis, permit monitoring susceptibility patterns and detecting resistant clinical isolates in an early stage (strong recommendation, moderate-quality evidence)20.
28. The consensus panel considers that automated AFSTs methods marketed in Colombia may be used to determine antifungal susceptibility (strong recommendation, moderate-quality evidence)20.
29. The consensus panel recommends not performing AFSTs for all Candida Spp. clinical isolates not associated with IFD on a routinely basis, unless no appropriate response to the antifungal therapy of choice is achieved, or a history of administration of an azole or an echinocandin exists, and should always be associated with clinical suspicion of therapeutic failure (strong recommendation, high-quality evidence)27.
Does the AFST result affect the choice of the antifungal therapy?
Recommendation
30. consensus panel considers that, even though the identification of the causative species of candidemia/ IC may predict its antifungal susceptibility profile, local epidemiological patterns may vary and affect its predictive value (strong recommendation, moderate-quality evidence)12.
31. The consensus panel considers that there is a relationship between sub-optimal use and dosing of antifungal therapy and changes in the distribution of yeast-like species and the onset of antifungal resistance (strong recommendation, high-quality evidence)20,21.
VII. What is the diagnostic value of serum biomarkers in the candidemia/IC management?
Recommendation
32. The consensus panel considers that serum biomarkers may be a supplemental tool that enhances the diagnostic performance, helps in the initiation of diagnostic-driven antifungal therapy, provide prognostic information and/or allow therapeutic monitoring in some difficult cases of IFD, even though their availability and highcost are significant limitations (weak recommendation, moderate-quality evidence)28,29.
33. The consensus panel considers that serum biomarkers may improve candidemia/IC diagnosis and prognosis when used serially in high-risk patients who have been hospitalized for a long time (weak recommendation, moderate-quality evidence) (Annex 11)30,31.
VIII. What is the diagnostic value of nucleic acid tests and mass spectrophotometry in the management of a candidemia/IC?
Recommendation
34. The consensus panel considers that fungal DNA detection tests including pan-fungal methods and speciesspecific detection methods are useful supplementary diagnostic tools, and yield results 1 day to 4 weeks earlier than conventional diagnostic methods (strong recommendation, moderate-quality evidence)28,32.
35. The consensus panel considers that direct identification of involved Candida species from positive blood culture bottles by PCR automated systems allows the identification of species within 1 - 2.5 hours (strong recommendation, moderate-quality evidence) (Annex 12)19,32.
36. The consensus panel considers that direct identification by PCR automated systems of involved Candida species from whole blood samples permits identification of species without the need to wait 1-2 days for blood culture bottles positive results (weak recommendation, lowquality evidence) (Annex 12)31,33-35.
37. The consensus panel considers that protenomic fingerprint mass spectrophotometry (MALDI-TOF MS) is a specific, robust, rapid and reproducible diagnostic tool for routine identification of different Candida species associated to an IFD (strong recommendation, highquality evidence) (Annex 12)3,36.
Antifungal prophylaxis for candidemia/IC
Doses in adult patients are established in the following recommendations. See (Table 8 for doses in pediatric patients.
IX. Is antifungal prophylaxis recommended for prevention of candidemia/IC? In which clinical settings is antifungal prophylaxis initiation recommended?
X. What is the standard practice according to the clinical setting?
HIV-Infected Patients
Recommendation
39. Initiation of antifungal prophylaxis is not recommended in HIV-infected patients (strong recommendation, low-quality evidence)37.
Solid organ transplant recipients
Renal transplantation
Recommendation
40. The initiation of antifungal prophylaxis is not recommended in patients with renal transplantation (strong recommendation, high-quality evidence)38.
Pancreas transplantation
Recommendation
41. A dose of FCZ (400 mg daily) for one week, is recommended in patients with pancreas transplantation, to reduce the risk of IC onset after the transplant (strong recommendation, moderate-quality evidence)39.
Liver transplantation
Recommendation
42. The consensus panel recommends In patients with liver transplantation the initiation of antifungal therapy with FCZ (200 mg daily) IV., until the patient is discharged, and continue with FCZ (200 mg daily) OA., for at least three months following liver transplantation (strong recommendation, moderate-quality evidence)40-42.
Patients in the intensive care unit
Recommendation
43. The initiation of antifungal prophylaxis in all patients admitted in ICU is not recommended (strong recommendation, moderate-quality evidence)43.
44. In patients with intraabdominal surgery at very high-risk of IC, in ICUs with IC incidence > 10%, the consensus panel considers that an institutional protocol of early antifungal or biomarker-driven treatment should be established, or antifungal prophylaxis with FCZ (200 mg daily) should be initiated (weak recommendation, low-quality evidence) (Table 3, Annex 11)43.
Hematopoietic stem cell transplantation (HCT) recipients
Recommendation
45. In HCT recipients, the consensus panel considers that the initiation of antifungal prophylaxis against Candida Spp. IFDs is required but does not exclude the need to initiate antifungal prophylaxis against IFDs caused by filamentous fungi, where indicated (strong recommendation, high-quality evidence).
46. In neutropenic HCT recipients, the consensus panel recommends initiating antifungal therapy with FCZ (400 mg daily) OA., if the initiation of antifungal prophylaxis against IFDs caused by fungi other than Candida Spp. is not being considered (weak recommendation, lowquality evidence)44,45.
47. In high-risk HCT recipients, the consensus panel considers that PCZ (suspension [200 mg 3 times daily] OA., or tablets [300 mg twice daily on day 1, then 300 mg daily]) administered as antifungal prophylaxis against filamentous fungi also offers suitable protection against Candida Spp. (strong recommendation, high-quality evidence) 44,45.
Hematological patients
Recommendation
48. In hematological patients, the consensus panel considers that antifungal prophylaxis against Candida Spp. IFDs should be initiated, without excluding the need to initiate antifungal prophylaxis against IFDs caused by filamentous fungi, where indicated (strong recommendation, high-quality evidence).
49. In neutropenic patients at high risk of infection, the consensus panel recommends considering antifungal prophylaxis as the standard of care (strong recommendation, moderate-quality evidence)46.
50. In induction or consolidation chemotherapy-receiving hematological patients for acute lymphoblastic leukemia (ALL), acute myeloblastic leukemia/myelodysplastic syndrome (AML/MDS), the consensus panel considers that PCZ (suspension [200 mg 3 times daily] OA., or tablets [300 mg twice daily on day 1, then 300 mg daily]), should be administered as antifungal prophylaxis against filamentous fungi, and also offers suitable protection against Candida Spp. In all patients, the appropriateness of antifungal prophylaxis should be determined on an individual basis, because of the interactions between chemotherapeutic agents and azole antifungal therapy (strong recommendation, moderate-quality evidence)47-49.
51. In long-term neutropenic patients at risk of IC, the consensus panel recommends initiating FCZ (400 mg daily) OA., until recovery from neutropenia (strong recommendation, moderate-quality evidence).
Candidemia/IC in non-neutropenic patients
Doses in adult patients are established in the following recommendations. See Table 8 for doses in pediatric patients.
XI. When should initiation of empirical antifungal therapy (EAFT) be considered in non-neutropenic patients? When is EAFT initiation recommended?
Recommendation
52. In non-neutropenic patients with clinical suspicion of IFD, EAFT should be initiated before the diagnostic confirmation (strong recommendation, moderate-quality evidence).
53. The consensus panel considers that the decision to initiate EAFT in non-neutropenic patients, in the absence of a recognized focus of infection, should be based on the clinical evaluation of risk factors, the results of IFD biomarkers and/or data from microbiological cultures (strong recommendation, moderate-quality evidence).
XII. What is the recommendation for choosing the type of drug, dose, and duration of EAFT in non-neutropenic patients?
Recommendation
54. In non-neutropenic patients, the consensus panel recommends including in the EAFT of choice an echinocandin (CAS [70 mg loading dose, then 50 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily]) (strong recommendation, moderate-quality evidence) 50-54.
55. The consensus panel considers that there are no differences between echinocandins in the clinical setting of non-neutropenic patients. The choice will depend on interactions with other drugs, liver failure, side effects and treatment costs (strong recommendation, moderatequality evidence)10,43,50-56.
56. FCZ (800 mg loading dose, then 400 mg daily) IV., is an acceptable alternative to EAFT for non-neutropenic, not critically ill, azole antifungal therapy-naïve patients unlikely to have azole-resistant isolates, according to the epidemiologic setting (strong recommendation, moderate-quality evidence)10,43,55,60.
57. AmB (AmB-D [0,7-1 mg/kg daily], AmB-L [3-5 mg/kg daily], is an acceptable alternative to EAFT against candidemia/IC for non-neutropenic patients, in case of limited availability and/or intolerance and/or documented antifungal resistance to other antifungal drugs of choice (strong recommendation, moderate-quality evidence)10,57-59.
58. The consensus panel recommends not a specific duration of EAFT for non-neutropenic patients; however, the consensus panel considers that the same recommendation as for a targeted antifungal therapy should be followed (strong recommendation, moderate-quality evidence)10,43,55.
XIII. Is the removal of central venous catheters (CVC) recommended for non-neutropenic patients with candidemia/IC? What is the recommendation for implanting a new CVC?
Recommendation
59. In non-neutropenic patients, the consensus panel recommends early removal of CVC, if there is evidence of infection and where the CVC is considered the infection source. The decision to remove the CVC should be made on an individual basis (strong recommendation, moderate-quality evidence) 55.
60. Early removal of CVC is recommended, if peripheral blood cultures or blood collected via the CVC are persistently positive or when the patient is clinically unstable. If candidemia persists, the removal or change of all endovascular accesses should also be considered (strong recommendation, moderate-quality evidence) 61-65.
61. In non-neutropenic patients, the consensus panel recommends implanting a new CVC in those with negative control blood cultures and without clinical signs of active infection, as necessary (strong recommendation, low-quality evidence) 61-65.
XIV. What is the role of other diagnostic methods when candidemia/IC is suspected in non-neutropenic patients?
Recommendation
62. In non-neutropenic patients is recommended an followup by performing blood cultures every 24-48 hours, in order to establish when documented mycological clearance of candidemia/IC occurs (strong recommendation, high-quality evidence) (Section: Diagnosis of Invasive Candidiasis [IC])10,66.
63. In non-neutropenic patients is recommended additional follow-up tests following the candidemia/IC diagnosis (strong recommendation, high-quality evidence) (Table 7) 10,66.
What is the role of ophthalmological examination?
Recommendation
64. The consensus panel recommends dilated ophthalmological examination in any non-neutropenic patient with a diagnosis of candidemia/IC preferably by an ophthalmologist, within the first week following IFD diagnosis (strong recommendation, high-quality evidence) (Table 7) 10,26,29,66,67.
What is the role of diagnostic imaging?
Recommendation
65. Ultrasound is considered an effective tool for the diagnosis of candidemia/IC-associated complications such as septic thrombosis, hepatic or splenic abscesses, and candidal endocarditis (strong recommendation, highquality evidence) (Table 7) 66,68.
66. In non-neutropenic patients, is recommended performing a hepatobiliary ultrasonography, a doppler ultrasonography of the jugular-subclavian CVC exit site, and an echocardiogram, if blood cultures are persistently positive or when clinical signs compatible with endocarditis exist (presence of cutaneous septic embolisms, de novo heart failure or new heart murmur) (strong recommendation, low-quality evidence) 66.
XV. When is therapy de-escalation recommended in nonneutropenic patients diagnosed with candidemia/IC?
Recommendation
67. The consensus panel recommends in non-neutropenic patients who initiated antifungal therapy with an echinocandin or AmB, implementing a therapy de-escalation scheme (after 5-7 days) to FCZ (400-800 mg daily) OA. or IV., if patients are clinically stable, apt for oral administration, azole antifungal therapy-naïve, and have FCZ-susceptible clinical isolates (strong recommendation, moderatequality evidence) (Table 4, Annex 9 and annex 10)10,55.
68. For documented IFDs caused by C. glabrata, the consensus panel recommends implementing a therapy deescalation scheme to higher-dose FCZ (800 mg daily) OA. or IV., or VCZ (400 mg twice daily for 2 doses, then 200-300 mg twice daily) OA. or IV., provided that clinical isolates are susceptible to FCZ and/or VCZ (strong recommendation, low-quality evidence)10,55.
Candidemia/IC in neutropenic patients
Doses in adult patients are established in the following recommendations. See Table 8 for doses in pediatric patients.
XVI. When should the initiation of EAFT be considered in neutropenic patients? When is EAFT initiation recommended?
Recommendation
69. It is recommended in neutropenic patients who have not initiated prophylaxis treatment with azoles, and have a clinical suspicion of IFD initiating EAFT before the diagnostic confirmation (strong recommendation, moderate-quality evidence).
70. In patients who have had neutropenia for more than 7 days and persistent fever, consideration should be given to candidemia/IC diagnosis, despite the use of broadspectrum antibiotics (strong recommendation, moderate-quality evidence).
XVII. What is the recommendation for choosing the type of drug, dose, and duration of EAFT in neutropenic patients?
Recommendation
71. In neutropenic patients with persistent fever, it is recommended considering EAFT as the standard of care and determining the appropriate antifungal therapy on an individual basis (strong recommendation, moderate-quality evidence).
72. The consensus panel recommends in any neutropenic patient, including an echinocandin in the EAFT of choice (CAS [70 mg loading dose, then 50 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily]) in patients on prophylaxis treatment with azoles and without suspected Invasive Aspergillosis (IA) (strong recommendation, moderate-quality evidence)50-54.
73. The consensus panel considers that there are no differences between echinocandins in the clinical setting of neutropenic patients. The choice will depend on interactions with other drugs, liver failure, side effects and treatment costs (strong recommendation, moderatequality evidence)10,43,50-56.
74. AmB (AmB-D [0,7-1 mg/kg daily], AmB-L [3-5 mg/kg daily], AmB-CL [3-5 mg/kg daily]), may be considered as an acceptable alternative to EAFT for candidemia/IC, but its nephrotoxic potential should be considered in neutropenic patients (strong recommendation, moderate-quality evidence)59,60.
75. FCZ (800 mg loading dose, then 400 mg daily) IV., is an acceptable alternative to EAFT for candidemia/IC, in neutropenic patients with persistent fever and severe mucositis, who have not received antifungal prophylaxis against Candida Spp. and at low-risk of IFD caused by mold (strong recommendation, moderate-quality evidence)10,59,43.
76. VCZ (6 mg/kg twice daily for 2 doses, then 3-4 mg/ kg twice daily) IV., is an acceptable alternative to EAFT when the clinical setting additionally requires anti-mold treatment (weak recommendation, moderate-quality evidence)69,70.
77. In neutropenic patients with suspected IC/candidemia due to azole- and/or echinocandin-resistant clinical isolates, the consensus panel recommends initiating antifungal therapy with AmB (AmB-D [0,7-1 mg/kg daily], AmB-L [3-5 mg/kg daily]) (strong recommendation, low-quality evidence)59,60.
78. In neutropenic patients, the recommended duration of antifungal therapy is two weeks, after control blood cultures are negative and symptoms attributable to IFD have resolved. In patients with metastatic foci or documented invasive infection longer antifungal therapy may be required (strong recommendation, moderate-quality evidence)10.
XVIII. Is the removal of CVC recommended in neutropenic patients with candidemia/IC? What is the recommendation for implanting a new CVC?
Recommendation
79. In neutropenic patients, the consensus panel recommends the early removal of CVC, if there is evidence of infection and where the CVC is considered the infection source. The decision to remove the CVC should be made on an individual basis (strong recommendation, moderate-quality evidence)55.
80. Early removal of CVC is recommended, if peripheral blood cultures or blood collected via the CVC are persistently positive or when the patient is clinically unstable. If candidemia persists, the removal or change of all endovascular accesses should also be considered (strong recommendation, moderate-quality evidence)10.
81. In neutropenic patients, the consensus panel recommends implanting a new CVC in patients with negative control blood cultures and without clinical signs of active infection, where appropriate (strong recommendation, low-quality evidence)61-65.
XIX. What is the role of other diagnostic methods when is suspected candidemia/IC in neutropenic patients?
Recommendation
82. In neutropenic patients is recommended an follow-up by collection of blood cultures every 24-48 hours, in order to establish when documented mycological clearance of candidemia/IC occurs (strong recommendation, high-quality evidence) (Section: Diagnosis of Invasive Candidiasis [IC])10,66.
83. In any neutropenic patient is recommended additional follow-up tests following candidemia/IC diagnosis (strong recommendation, high-quality evidence) (Table 7)10,66
What is the role of ophthalmological examination?
Recommendation
84. The consensus panel recommends dilated ophthalmological examination in any neutropenic patient with a diagnosis of candidemia/IC preferably by an ophthalmologist, within the first week following IFD diagnosis (strong recommendation, high-quality evidence) (Table 7)10,26,66.
85. In febrile neutropenic patients, is recommended performing dilated ophthalmological examination following neutrophil recovery (count > 500 cells/mm3 ) (strong recommendation, low-quality evidence)10,26,29,66.
What is the role of diagnostic imaging?
Recommendation
86. Ultrasound is considered an effective tool for the diagnosis of candidemia/IC-associated complications such as septic thrombosis, hepatic or splenic abscesses, and candidal endocarditis (strong recommendation, highquality evidence) (Table 7)10,66.
87. In neutropenic patients, is recommended performing a hepatobiliary ultrasonography, a doppler ultrasonography of the jugular-subclavian CVC exit site, and an echocardiogram, if blood cultures are persistently positive or when clinical signs compatible with endocarditis exist (presence of cutaneous septic embolisms, de novo heart failure or new heart murmur) (strong recommendation, low-quality evidence)10,66.
XX. When is therapy de-escalation recommended in neutropenic patients with candidemia?
Recommendation
88. In neutropenic patients, is recommended a therapy deescalation scheme (after 5-7 days) from an echinocandin to FCZ (400-800 mg daily) OA. or IV., if patients are clinically stable, apt for oral administration, azole antifungal therapy-naïve, and have FCZ-susceptible clinical isolates (strong recommendation, moderate-quality evidence) (Table 4, Annex 9 and Annex 10) 10,55.
89. In neutropenic patients, is recommended a therapy deescalation scheme (after 5-7 days) from AmB to FCZ (400-800 mg daily) OA. or IV., if patients are clinically stable, apt for oral administration, azole antifungal therapy-naïve, and have FCZ-susceptible clinical isolates (strong recommendation, moderate-quality evidence) (Table 4, Annex 9 and 10)10,55,71,72.
90. For documented IFDs caused by C. glabrata, the consensus panel recommends implementing a therapy deescalation scheme to higher-dose FCZ (800 mg daily) OA. or IV., or VCZ (400 mg twice daily for 2 doses, then 200-300 mg twice daily) OA. or IV., provided that clinical isolates are susceptible to FCZ and/or VCZ (strong recommendation, low-quality evidence)10,55.
91. VCZ (400 mg twice daily for 2 doses, then 200 mg twice daily) OA. or IV., may be used in a therapy de-escalation scheme during the neutropenic phase, if the patient is clinically stable, apt for oral administration, and has VCZ-susceptible clinical isolates (weak recommendation, low-quality evidence)10,71.
Targeted antifungal therapy for Candidemia/IC
Doses in adult patients are established in the following recommendations. See Table 8 for doses in pediatric patients.
XXI. What is the recommendation for choosing the type of drug, dose, and duration of targeted antifungal therapy according to the risk population?
Recommendation
92. The consensus panel considers that in any patient with suspected or microbiologically proven IC/candidemia, targeted antifungal therapy should be initiated (strong recommendation, high-quality evidence)12,38,43,73.
93. A Candida Spp. isolation in a single culture of peripheral blood or blood collected via the CVC is considered as proven IC/candidemia (strong recommendation, high-quality evidence) (Annex 5 Anexo 6, anexo 7)12,38,43.
Non-neutropenic patients and/or patients in critical condition with proven candidemia/IC
94. In any non-neutropenic patient and/or patient in critical condition, the consensus panel recommends including in the antifungal therapy of choice an echinocandin (CAS [70 mg loading dose, then 50 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily]) (strong recommendation, moderate-quality evidence) (Table 8, Annex 13 and 14)10,11,66.
95. The consensus panel considers that there are no differences between echinocandins in the clinical setting of non-neutropenic patients and/or patients in critical condition. The choice will depend on interactions with other drugs, liver failure, side effects and treatment costs (strong recommendation, moderate-quality evidence)11,74,75.
96. FCZ (800 mg loading dose, then 400 mg daily) IV. and VCZ (6 mg/kg twice daily for 2 doses, then 4 mg/kg twice daily) IV., are acceptable alternative treatments for clinically stable, azole antifungal therapy-naïve patients with FCZ- and/or VCZ-susceptible clinical isolates (strong recommendation, moderate-quality evidence)11,74,76.
97. Lipid formulations of AmB (AmB-L [3-5 mg/kg day], AmB-CL [3-5 mg/kg daily]), may be considered if the CNS is affected, endocarditis occurs, the patient experiences side effects or the etiological agent isolate shows antifungal resistance to echinocandins. In patients in ICUs, the consensus panel does not recommend the use of AmB-D (strong recommendation, moderatequality evidence) (Table 8)10,11,66.
98. For clinical isolates suspected to be azole- or echinocandin-resistant, the consensus panel recommends initiating antifungal therapy with AmB-L (3-5 mg/kg daily) (strong recommendation, low-quality evidence) (Table 4, Annex 9 and 10)10-12,66,74.
99. For documented IFDs caused by C. krusei, the consensus panel recommends initiating antifungal therapy with an echinocandin, AmB or VCZ (strong recommendation, low-quality evidence) (Annex 9 and Annex 14)43,77,78.
100. The consensus panel recommends performing azole and/or echinocandin AFST for clinical isolates from sterile sites, particularly in patients who have previously received antifungal therapy with azoles and/or echinocandins, or in patients with a documented IFDs caused by C. glabrata or C. parapsilosis (strong recommendation, moderate-quality evidence) (Annex 9 and 10)43,77,78.
101. The recommended duration of antifungal therapy in non-neutropenic patients and/or patients in critical condition, without metastatic complications, is two weeks after control blood cultures are negative and symptoms attributable to IFD have resolved (strong recommendation, moderate-quality evidence) (Section: Candidemia/IC in Non-Neutropenic Patients)74,78.
102. In non-neutropenic patients and/or patients in critical condition, the consensus panel recommends a therapy de-escalation scheme (after 5-7 days) from an echino candin to FCZ (400-800 mg daily) OA. or IV., if patients are clinically stable, apt for oral administration, azole antifungal therapy-naïve, and have FCZ-susceptible clinical isolates (strong recommendation, moderatequality evidence) (Table 4, Annex 9 and 10) (Section: Candidemia/IC in Non-Neutropenic Patients) 74,78.
103. In non-neutropenic patients and/or patients in critical condition, the consensus panel recommends a therapy de-escalation scheme (after 5-7 days) from AmB to FCZ (400-800 mg daily) OA. or IV., or VCZ (400 mg twice daily for 2 doses, then 200 mg twice daily) OA. or IV., if patients are clinically stable, apt for oral administration, azole antifungal therapy-naïve, and have FCZ- and/or VCZsusceptible clinical isolates (strong recommendation, moderate-quality evidence) (Table 4, Annex 9 and 10) (Section: Candidemia/IC in Non-Neutropenic Patients) 74,78.
104. For documented IFDs caused by C. glabrata, the consensus panel recommends implementing a therapy deescalation scheme to higher-dose FCZ (800 mg daily) OA. or IV., or VCZ (400 mg twice daily for 2 doses, then 200-300 mg twice daily) OA. or IV., provided that clinical isolates are susceptible to FCZ and/or VCZ (strong recommendation, low-quality evidence)74,78.
105. The consensus panel considers that the CVC may be retained in patients receiving antifungal therapy with an echinocandin or AmB-L, if it is established that the CVC is necessary, the CVC is not the source of infection or the documented IFD is not caused by C. parapsilosis. If the patient does not respond to the treatment (after 3 to 5 days) the removal of CVC should be considered. The decision to remove the CVC should be made on an individual basis (strong recommendation, moderatequality evidence) (Section: Candidemia/IC in NonNeutropenic Patients)11,66.
106. The consensus panel recommends the use of diagnostic techniques to monitor the response to treatment, such as control blood cultures, until negative results are obtained and ophthalmological examination and transesophageal echocardiography, where necessary (strong recommendation, moderate-quality evidence) (Table 7) (Section: Candidemia/IC in Non-Neutropenic Patients)10-12,43,66.
Neutropenic patients with proven candidemia/IC
107. In any neutropenic patients, the consensus panel recommends in the antifungal therapy of choice an echinocandin (CAS [70 mg loading dose, then 50 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily])in patients on prophylaxis treatment with azoles and without suspected IA (strong recommendation, moderate-quality evidence) (Table 8, Annex 13 and Annex 14)10-12,43,66.
108. The consensus panel considers that there are no differences between echinocandins in the clinical setting of neutropenic patients. The choice will depend on interactions with other drugs, liver failure, side effects and treatment costs (strong recommendation, moderatequality evidence)74,75,78.
109. AmB (AmB-D [0,7-1 mg/kg daily], AmB-L [3-5 mg/kg daily], AmB-CL [3-5 mg/kg daily]), may be considered as an acceptable alternative treatment for neutropenic patients, but its nephrotoxic potential should be considered (strong recommendation, moderate-quality evidence) (Table 8)10,11,66,74.
110. FCZ (800 mg loading dose, then 400 mg daily) IV., is an acceptable alternative treatment for neutropenic patients who are not in critical condition, azole antifungal therapynaïve, and have FCZ-susceptible clinical isolates (weak recommendation, moderate-quality evidence)10,11,66,74.
111. FCZ (400 mg [6 mg/kg] daily), may be used as maintenance treatment in patients with persistent neutropenia who are clinically stable, and with FCZ-susceptible clinical isolates and negative control blood cultures (weak recommendation, moderate-quality evidence)10,11,74.
112. VCZ (6 mg/kg twice daily for 2 doses, then 4 mg/kg twice daily) IV., may be used in patients when anti-mold treatment is additionally required because of the clinical setting (weak recommendation, moderate-quality evidence)10,11,74.
113. VCZ (6 mg/kg twice daily for 2 doses, then 4 mg/kg twice daily) IV., may be used as maintenance treatment in patients with persistent neutropenia who are clinically stable, and with VCZ-susceptible clinical isolates and negative control blood cultures (weak recommendation, moderate-quality evidence)10,11,74.
114. For clinical isolates suspected to be azole- or echinocandin-resistant, the consensus panel recommends initiating antifungal therapy with AmB-L (3-5 mg/kg daily) (strong recommendation, low-quality evidence) (Table 4, Annex 9 and Annex 10)10-12,43,66.
115. For documented IFDs caused by C. krusei, the consensus panel recommends initiating antifungal therapy with an echinocandin, AmB or VCZ (strong recommendation, low-quality evidence) (Annex 9 and 14) 74,78.
116. The consensus panel recommends performing azole and/or echinocandin AFST for clinical isolates from sterile sites, particularly in patients who have previously received antifungal therapy with azoles and/or echinocandins, or in patients with a documented IFDs caused by C. glabrata or C. parapsilosis (strong recommendation, moderate-quality evidence) (Annex 9 and 10)74,78.
117. The recommended duration of antifungal therapy in neutropenic patients, without metastatic complications, is two weeks after control blood cultures are negative and symptoms attributable to IFD have resolved. Patients with chronic disseminated candidiasis (CDC) may require longer antifungal therapy (strong recommendation, moderate-quality evidence) (Section: Candidemia/IC in Neutropenic Patients)74,78.
118. In neutropenic patients, therapy de-escalation scheme (after 5-7 days) from an echinocandin to FCZ (400-800 mg daily) OA. or IV., or VCZ (400 mg twice daily for 2 doses, then 200 mg twice daily) OA. or IV. is recommended, if patients are clinically stable, have recovered from neutropenia, are apt for oral administration, azole antifungal therapy-naïve, and have FCZ- and/or VCZsusceptible clinical isolates (strong recommendation, moderate-quality evidence) (Section: Candidemia/IC in Neutropenic Patients)74,78.
119. In neutropenic patients, therapy de-escalation scheme (after 5-7 days) from AmB to FCZ (400-800 mg daily) OA. or IV., or VCZ (400 mg twice daily for 2 doses, then 200 mg twice daily) OA. or IV. is recommended, if patients are clinically stable, have recovered from neutropenia, are apt for oral administration, azole antifungal therapy-naïve, and have FCZ- and/or VCZ-susceptible clinical isolates (strong recommendation, moderatequality evidence) (Section: Candidemia/IC in Neutropenic Patients)74,78.
120. The consensus panel recommends early CVC removal, where this is not possible, initiation of antifungal therapy with an echinocandin or AmB-L is recommended, if it has been established that the CVC is not the infection source or the documented IFD is not caused by C. parapsilosis. If the patient does not respond to the treatment (after 3 to 5 days) CVC removal should be considered (strong recommendation, moderate-quality evidence) (Section: Candidemia/IC in Neutropenic Patients)74,78.
121. Sources of infection different than the CVC (e.g. gastrointestinal tract) should be considered in neutropenic patients. The decision to remove the CVC should be made on an individual basis (strong recommendation, moderate-quality evidence) (Section: Candidemia/IC in Neutropenic Patients)74,78.
122. The consensus panel recommends the use of diagnostic techniques to monitor the response to treatment, such as control blood cultures, until negative results are obtained and ophthalmological examination and transesophageal echocardiography, where necessary. In patients with CVC, the consensus panel recommends a doppler ultrasonography of the jugular-subclavian CVC exit site (strong recommendation, moderate-quality evidence) (Table 7) (Section: Candidemia/IC in Neutropenic Patients)74,78.
123. The consensus panel recommends dilated ophthalmological examination, within the first week after recovery from neutropenia, because ophthalmological findings of choroidal infection are minimal until neutrophil recovery (strong recommendation, low-quality evidence) (Section: Candidemia/IC in Neutropenic Patients)74,78.
XXII. What is the recommendation for choosing a combined antifungal therapy according to the risk population?
Recommendation
124. In patients with candidemia/IC, the consensus panel recommends not the initiation of combined antifungal therapy, except when they are clinical isolates, which are considered multiresistant, and / or are emerging yeasts such as C. auris, and always under specific considerations (strong recommendation, moderate-quality evidence) (Annex 14)79,80.
Candidemia/IC in neonate patients
XXV. How is candidemia/IC diagnosed in neonate patients?
Recommendation
127. In any neonate patient with clinical suspicion of candidemia/IC, the consensus panel recommends performing serial blood cultures and urine cultures (strong recommendation, low-quality evidence)84.
128. In any neonate patient with blood cultures and/or urine cultures positive for Candida Spp., is recommended performing additional lumbar puncture and ophthalmologic examination (strong recommendation, lowquality evidence)10.
129. The consensus panel recommends in any neonate patient, with persistently positive blood cultures (72 hours after antifungal therapy initiation), additionally perform an echocardiogram, an ultrasonography of the brain and/or a CAT of the genitourinary tract, liver and spleen (strong recommendation, low-quality evidence)10,84.
130. The consensus panel recommends in any neonate patient with clinical suspicion of candidemia/IC, performing serial blood cultures until documented mycological clearance and symptoms attributable to IFD have resolved (strong recommendation, low-quality evidence)84.
131. The consensus panel recommends in any neonate patient with persistent candidemia on the 7th day of antifungal therapy, performing imaging of CNS and bones (strong recommendation, low-quality evidence)84.
XXVI. What is the standard practice in neonate patients with candidemia/IC?
Recommendation
132. The consensus panel recommends in neonate patients with candidemia/IC, initiating antifungal therapy with AmB-D (1 mg/kg daily) (strong recommendation, moderate-quality evidence) (Table 8)10.
133. FCZ (25 mg/kg daily, then 12 mg/kg daily) OA. or IV., is an acceptable alternative antifungal therapy for azole antifungal therapy-naïve patients with FCZ-susceptible clinical isolates (strong recommendation, moderatequality evidence) (Table 4), (Annex 9 and 10)10.
134. AmB-L (3-5 mg/kg daily), may be considered as an alternative antifungal therapy but should be used with caution, particularly when the urinary tract is compromised (weak recommendation, low-quality evidence) (Annex 13)10.
135. The consensus panel recommends in neonate patients diagnosed with candidemia/IC, the removal of CVC (strong recommendation, moderate-quality evidence)10.
136. In neonate patients, without metastatic complications, the recommended duration of antifungal therapy is two weeks, after control blood cultures are negative and symptoms attributable to IFD have resolved (strong recommendation, low-quality evidence)10.
137. In patients with metastatic complications or in special clinical situations, longer antifungal therapy may be required (strong recommendation, low-quality evidence)10.
XXVII. What is the standard practice in neonate patients with CNS infection?
Recommendation
138. The consensus panel recommends in neonate patients diagnosed with candidal meningitis, initiating antifungal therapy with AmB-D (1 mg/kg daily) (strong recommendation, low-quality evidence) (Table 8, Annex 13)10.
139. AmB-L (5 mg/kg daily), may be considered as an alternative antifungal therapy for neonate patients with candidal meningitis (strong recommendation, lowquality evidence) (Table 8, Annex 13)10.
140. 5-FC (25 mg/kg 4 times daily), alone or in combination, may be considered as an antifungal salvage treatment in neonate patients without appropriate clinical response to AmB, but its adverse effects should be considered (weak recommendation, low-quality evidence10.
141. The consensus panel recommends in neonate patients implementing a therapy de-escalation scheme, after negative cultures, from AmB to FCZ (12 mg/kg daily) OA. or IV., if patients are clinically stable, apt for oral administration, and have FCZ-susceptible clinical isolates (strong recommendation, low-quality evidence) (Table 4, Annex 9 and 10)10.
142. The consensus panel considers that antifungal therapy duration will depend on the resolution of all signs, symptoms, and CSF and radiological abnormalities (strong recommendation, low-quality evidence)10.
143. The consensus panel recommends in these patients removing infected CNS devices (e.g. ventriculostomy drains, shunts, stimulators, and chemotherapy ports) (strong recommendation, low-quality evidence)10.
XXVIII. What is the recommendation for choosing the antifungal therapy for newborns according to the clinical setting?
Recommendation
144. AmB-D (1 mg/kg daily), may be considered as an antifungal therapy in neonate patients with candidemia/CI (strong recommendation, moderate-quality evidence) (Table 8, Annex 13)10.
145. AmB-L (3-5 mg/kg daily), may be considered as an acceptable alternative antifungal therapy but should be used with caution, particularly when the urinary tract is compromised (weak recommendation, low-quality evidence)10.
146. FCZ (25 mg/kg daily, then 12 mg/kg daily) OA. or IV., is an acceptable alternative antifungal therapy for azole antifungal therapy-naïve patients with FCZ-susceptible clinical isolates (strong recommendation, moderatequality evidence) (Table 4, Annex 9 and Annex 10)10.
147. The consensus panel recommends in patients with neonatal candidiasis (CNEO) implementing a therapy deescalation scheme (after 3-5 days) from AmB to FCZ (12 mg/kg daily) OA. or IV., if patients are clinically stable, apt for oral administration, and have FCZ-susceptible clinical isolates (strong recommendation, low-quality evidence) (Table 4, Annex 9 and 10)10.
148. The consensus panel considers that in patients with CNEO, echinocandins (CAS [25 mg/m2 daily or 2 mg/kg daily], MIC [4-10 mg/kg daily]) should be used with caution and generally be limited to salvage antifungal therapy or in clinical situations in which AmB-D or FCZ are contraindicated (weak recommendation, low-quality evidence)10,85,86.
149. FCZ (12 mg/kg daily) is the antifungal therapy of choice for neonate patients with Candida Spp. urinary tract infection (weak recommendation, low-quality evidence) 87.
XXIX. Is antifungal prophylaxis recommended for neonate patients? In which clinical situations is antifungal prophylaxis initiation recommended?
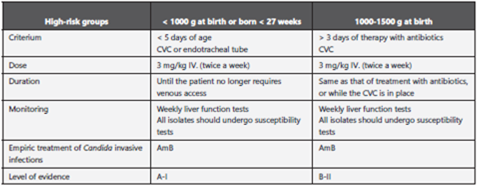
150. The consensus panel recommends in neonate patients weighing < 1000 g in NICUs with an IC incidence > 10%, initiating antifungal prophylaxis with FCZ (3-6 mg/kg, twice a week, for 6 weeks) (strong recommendation, high-quality evidence) (Annex 17)88-98.
151. Nystatin (100,000 IU 4 times daily, for 6 weeks) OA., is an alternative antifungal therapy for neonate patients weighing < 1500 g in situations in which FCZ is not available or clinical isolates are azole-resistant (weak recommendation, low-quality evidence)90,99-101.
Management of candidemia/IC in special situations
Doses in adult patients are established in the following recommendations. See Table 8 for doses in pediatric patients.
XXX. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy in pregnant patients?
Recommendation
152. In pregnant women, AmB (AmB-D [0.7-1 mg/kg daily], AmB-L [3-5 mg/kg daily]) is considered the antifungal therapy of choice for IC, but data available are insufficient to recommend other lipid formulations (strong recommendation, moderate-quality evidence) (Table 8, Annex 13 and 14 Annex 14-102-104.
153. In pregnant women, especially during the first trimester, antifungal therapy with azoles should be avoided, because there is possibility of congenital defects (strong recommendation, moderate-quality evidence)105,106.
154. Antifungal therapy with echinocandins is not recommended during pregnancy, because the available data on its use in this particular population of patients is insufficient (strong recommendation, low-quality evidence)107.
155. Antifungal therapy with 5-FC is not recommended during pregnancy, because of fetal abnormalities observed in several studies, and available data on its use in this particular population of patients is insufficient (strong recommendation, low-quality evidence)104.
XXXI. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for candidal chorioretinitis without vitritis?
Recommendation
156. The consensus panel recommends in patients with candidal chorioretinitis without vitritis, initiating antifungal therapy with FCZ (800 mg loading dose, then 400-800 mg daily) IV., or VCZ (6 mg/kg twice daily for 2 doses, then 4 mg/kg twice daily) IV., in azole treatment-naive patients with FCZ- and/or VCZ-susceptible clinical isolates (strong recommendation, moderate-quality evidence) (Table 8, Annex 13 and Annex 14)108-115.
157. The consensus panel recommends for clinical isolates with suspected or documented resistance to FCZ/VCZ, initiating antifungal therapy with AmB-L (3-5 mg/kg daily), with or without 5-FC (25 mg/kg 4 times daily) OA. (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)108,109,116.
158. The consensus panel recommends in patients with macular involvement, in addition to the above mentioned antifungal agents administrating intravitreal injection of AmB-D (5-10 μg in 0.1 mL sterile water) or VCZ (100 μg in 0.1 mL sterile water or normal saline) (strong recommendation, low-quality evidence)117-120.
159. The duration of antifungal therapy should depend on the resolution of the lesions, as determined by serial ophthalmological examinations, and should be at least 4-6 weeks (strong recommendation, low-quality evidence)109,121.
XXXII. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for candidal chorioretinitis with vitritis?
Recommendation
160. The consensus panel recommends in patients with candidal chorioretinitis with vitritis, initiating antifungal therapy with FCZ (800 mg loading dose, then 400-800 mg daily) IV., or VCZ (6 mg/kg twice daily for 2 doses, then 4 mg/kg twice daily) IV., in azole treatment-naïve patients with FCZ- and/or VCZ-susceptible clinical isolates. The consensus panel recommends administrating additional intravitreal injection of AmB-D (5-10 μg in 0.1 mL sterile water) or VCZ (100 μg in 0.1 mL sterile water or normal saline) (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)120-122.
161. The consensus panel recommends in patients with candidal chorioretinitis with vitritis, considering vitrectomy in order to decrease the burden of microorganisms, and to allow the removal of fungal abscesses that are inaccessible to systemic antifungal agents (strong recommendation, low-quality evidence)121.
162. The duration of antifungal therapy should depend on the resolution of the lesions, as determined by serial ophthalmological examinations, and should be at least 4-6 weeks (strong recommendation, low-quality evidence)109,121.
XXXIII. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for hepatosplenic candidiasis (HSC)?
Recommendation
163. The consensus panel recommends in patients with HSC, initiating antifungal therapy with AmB-L (3-5 mg/kg daily) or an echinocandin (CAS [70 mg loading dose, then 50 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily]), with a duration of antifungal therapy of two weeks, after control blood cultures are negative and symptoms attributable to IFD have resolved (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)123-131.
164. In patients with HSC, is recommended that consolidation antifungal therapy be continued with FCZ (400-800 mg daily), for azole treatment-naive patients with FCZ-susceptible clinical isolates (strong recommendation, lowquality evidence) (Table 8, Annex 13and Annex 14)128-130.
165. The duration of antifungal therapy should depend on the resolution of the lesions, with periodical imaging monitoring, which usually takes several months. Premature interruption of antifungal therapy may lead to relapse (strong recommendation, low-quality evidence)132,133.
XXXIV. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for candidal meningitis?
Recommendation
166. In patients with candidal meningitis, the consensus panel recommends initiating antifungal therapy with AmBL (3-5 mg/kg daily) IV., with or without 5-FC (25 mg/ kg 4 times daily) OA. (strong recommendation, lowquality evidence) (Table 8, Annex 13 and Annex 14)131,134-138.
167. AmB-D (0.7-1 mg/kg daily), with or without 5-FC (25 mg/kg 4 times daily) OA., may be considered as an alternative antifungal therapy in situations in which AmB-L is not available (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)134-137.
168. In patients with candidal meningitis, the recommended consolidation antifungal therapy is FCZ (400-800 mg daily [6-12 mg/kg daily]), for azole treatment-naive patients with FCZ-susceptible clinical isolates, following clinical improvement and documented mycological clearance (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)138,139.
169. The consensus panel recommends in these patients removing infected CNS devices (e.g. ventriculostomy drains, shunts, stimulators, and chemotherapy ports) (strong recommendation, low-quality evidence)140,141.
170. The consensus panel recommends that when infected CNS devices may not be removed, or in patients unresponsive to systemic antifungal therapy, considering intrathecal or intraventricular administration of AmB-D (0.01-1 mg in 2 mL 5% dextrose in distilled water) (strong recommendation, low-quality evidence)139,142-144.
XXXV. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for native valve and prosthetic valve candidal endocarditis?
Recommendation
171. The consensus panel recommends in patients with native valve candidal endocarditis, initiating antifungal therapy with AmB-L (3-5 mg/kg daily) with or without 5-FC (25 mg/kg 4 times daily) OA., or a high-dose echinocandin (CAS [150 mg daily], ANI [200 mg daily], MIC [150 mg daily]) (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)68,145-154.
172. In patients with native valve candidal endocarditis, the recommended consolidation antifungal therapy is FCZ (400-800 mg [6-12 mg/kg] daily), for at least 6 months, for azole treatment-naive patients with FCZ-susceptible clinical isolates, following clinical improvement and documented mycological clearance (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)152 .
173. The consensus panel recommends in patients with native valve candidal endocarditis, performing valve replacement surgery at treatment initiation, and continuing with antifungal therapy for at least 6 weeks after the surgery (strong recommendation, low-quality evidence)153-155.
174. The consensus panel recommends in patients in who valve replacement surgery is contraindicated, the administration of life-long antifungal therapy with FCZ (400- 800 mg [6-12 mg/kg] daily) in azole treatment-naïve patients with FCZ-susceptible clinical isolates (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)153-155.
175. The consensus panel recommends that patients with prosthetic valve endocarditis, follow the same regimens and interventions as those for patients with native valve endocarditis (strong recommendation, low-quality evidence)153-155.
176. The consensus panel recommends in patients diagnosed with infection of implantable cardiac devices or suppurative thrombophlebitis, initiating antifungal therapy with a high-dose echinocandin (CAS [150 mg daily], ANI [200 mg daily], MIC [150 mg daily]), followed by chronic suppressive therapy with FCZ (400-800 mg [6-12 mg/ kg] daily) (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)126,153-155.
XXXVI. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for Candida Spp. osteomyelitis and/or bone infections?
Recommendation
177. The consensus panel recommends in patients with Candida Spp. osteomyelitis and bone infections, initiating antifungal therapy with FCZ (400 mg [6 mg/kg] daily), for 6 to12 months provided that clinical isolates are susceptible to FCZ; or implementing an initial scheme with an echinocandin (CAS [70 mg loading dose, then 50 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily]), for 2 weeks followed by FCZ (400 mg daily), for 6 to 12 months (strong recommendation, low-quality evidence) (Table 8, Annex 13 and Annex 14)156-160.
178. In patients with Candida Spp. osteomyelitis or bone infections, the recommended duration of antifungal therapy is at least 6 months (strong recommendation, low-quality evidence)156-160.
179. The consensus panel recommends in patients with Candida Spp. osteomyelitis or bone infections, considering surgical debridement and removal of osteosynthesis material on an individual basis (strong recommendation, lowquality evidence) 161-163.
XXXVII. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for esophageal candidiasis (EFC) and recurrent EFC?
Recommendation
180. The consensus panel recommends in patients with EFC initiating antifungal therapy with FCZ (200-400 mg [3-6 mg/kg] daily) OA., for 14 to 21 days (strong recommendation, high-quality evidence) (Table 8, Annex 13 and Annex 14)164-173.
181. The consensus panel recommends in patients with EFC, who do not tolerate oral administration, initiating antifungal therapy with FCZ (400 mg [6 mg/kg] daily) IV. (strong recommendation, high-quality evidence) (Table 8, Annex 13 and Annex 14)179-173.
182. The consensus panel recommends in patients with FCZrefractory EFC, the administration of ITZ (200 mg daily), oral solution, or VCZ (6 mg/kg twice daily for 2 doses, then 3 mg/kg twice daily) OA., for 14 to 21 days (strong recommendation, high-quality evidence)170-171.
183. The consensus panel recommends in patients who cannot receive azole treatment, using echinocandins (CAS [70 mg loading dose, then 50 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily]), as an alternative antifungal therapy (strong recommendation, high-quality evidence) (Table 8, Annex 13 and Annex 14) 172-175.
184. The consensus panel recommends in patients with recurrent EFC, initiating suppressive antifungal therapy with FCZ (100-200 mg daily) three times a week (strong recommendation, high-quality evidence)176.
185. The consensus panel recommends in patients with recurrent EFC and diagnosed with HIV infection, initiating antiretroviral treatment (strong recommendation, high-quality evidence)165.
186. For recurrent EFC episodes, are recommended mycological cultures in order to identify the species and AFST to effectively guide the antifungal therapy (strong recommendation, low-quality evidence) (Annex 9)164,165.
XXXVIII.What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for candidal vulvovaginitis (CVV) and recurrent CVV?
Recommendation
187. The consensus panel recommends in patients with uncomplicated CVV, initiating antifungal therapy with any topical antifungal agent for 1-3 days, or initiating with single dose FCZ (150 mg daily) OA. (strong recommendation, high-quality evidence) (Table 8, Annex 13 and 14)177-180.
188. The consensus panel recommends in patients with complicated CVV, initiating antifungal therapy with FCZ (150 mg/72 h) OA. three doses, or with topical azole for 7 days (strong recommendation, high-quality evidence)180,181.
189. The consensus panel recommends for recurrent CVV episodes, mycological cultures in order to identify the species and AFST to effectively guide the antifungal therapy (strong recommendation, low-quality evidence) (Annex 9).
190. The consensus panel recommends in patients with recurrent CVV, with azole-susceptible clinical isolates, initiating induction antifungal therapy with a topical azole for 10 to 14 days, followed by FCZ (150 mg for week) OA., for 6 months (strong recommendation, high-quality evidence)182,184.
191. The consensus panel recommends in patients with documented C. glabrata CVV, administrating topical intravaginal boric acid (600 mg daily) for 2 weeks, or nystatin intravaginal ovules (100,000 U/day) for 2 weeks (strong recommendation, high-quality evidence)185,186.
XXXIX. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for candidal prostatitis (CP)?
Recommendation
192. The consensus panel recommends in patients with CP, with FCZ-susceptible clinical isolates, initiating antifungal therapy with FCZ (800 mg loading dose, then 400 mg daily) OA. for 6 weeks (strong recommendation, lowquality evidence) (Table 8, Annex 13 and 14)187,190.
193. The consensus panel recommends in patients with PC and FCZ-resistant clinical isolates, initiating antifungal therapy with AmB (AmB-D [0,7-1 mg/kg daily], AmB-L [3-5 mg/kg daily]) (strong recommendation, lowquality evidence) (Table 8, Annex 13 and Annex 14)187,190.
194. The consensus panel recommends in these patients considering supplemental surgical procedures, such as abscess drainage or transurethral resection of the prostate (strong recommendation, low-quality evidence)190.
XL. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy in patients with kidney failure?
Recommendation
195. The consensus panel recommends in patients with renal failure and a diagnosis of candidemia / IC, with a antifungal treatment with an azole, to adjust the initial doses (strong recommendation, high-quality evidence) (Table 8, Annex 13)80,81,191.
196. The consensus panel recommends adjusting azole dosing according to the creatinine clearance value and/ or the type of received renal replacement therapy, in patients with kidney failure diagnosed with candidemia/ IC (strong recommendation, high-quality evidence) (Table 8, Annex 13)80,81,191.
XLI. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy in patients undergoing renal replacement therapy?
XLII. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy in patients with liver failure or acute/chronic hepatic diseases?
Recommendation
198. The use of azoles for antifungal therapy of candidemia/ IC in patients with liver failure or acute/chronic hepatic diseases is not recommended (strong recommendation, high-quality evidence) (Table 8, Annex 13)80,81.
199. Echinocandins (CAS [70 mg loading dose, then 35 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily]) are recommended, as antifungal therapy for candidemia/IC, in patients with liver failure or acute/chronic hepatic diseases (strong recommendation, high-quality evidence) (Table 8, Annex 13) 80,192,193.
XLIII. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy in patients with circulatory assist devices?
XLIV. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy in patients with hypoalbuminemia?
Recommendation
201. The consensus panel recommends not adjusting the dose of antifungal agents during candidemia/IC treatment in patients with hypoalbuminemia (strong recommendation, low-quality evidence)194-197.
Intraabdominal/peritoneal IC
Recommendation
Doses in adult patients are established in the following recommendations. See Table 8 for doses in pediatric patients.
XLV. Do patients with a Candida Spp. isolate from an abdominal sample require antifungal therapy?
Recommendation
202. Initiation of targeted antifungal therapy in patients with a Candida Spp. isolate from an abdominal sample is not recommended. Isolates should be analyzed to distinguish between contamination, colonization, and infection based on the anatomical site and type of lesion, history of interventions, previous microbiological isolates and clinical setting of the patient. Antifungal therapy is not recommended for colonization or contamination isolates (strong recommendation, highquality evidence)198-202.
203. Targeted antifungal therapy should be initiated, in patients with clinical evidence of intraabdominal infection with a Candida Spp. isolate from an intraoperative abdominal sample or from drains placed within 24 hours (strong recommendation, high-quality evidence)13,202-204.
204. Targeted antifungal therapy should be initiated in patients with sepsis, septic shock or spontaneous intestinal perforation and a Candida Spp. clinical isolate from an intraabdominal sample (strong recommendation, moderate-quality evidence)14,202-205.
205. The consensus panel considers that targeted antifungal therapy, in patients with yeast-like isolates from swabs of superficial wounds or drainages that have been in place ≥ 24 hours should not be initiated (strong recommendation, moderate-quality evidence)199.
XLVI. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy for Candida Spp. abdominal sepsis?
Recommendation
206. The consensus panel recommends including in the antifungal therapy of choice an echinocandin (CAS [70 mg loading dose, then 50 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily]), in patients with Candida Spp. abdominal sepsis (strong recommendation, moderate-quality evidence) (Table 8, Annex 13)and Annex 14)43,206.
207. The consensus panel considers that there are no differences between echinocandins in the clinical setting of patients with abdominal sepsis. The choice will depend on interactions with other drugs, liver failure, side effects and treatment costs (strong recommendation, moderate-quality evidence)11,74,75.
208. FCZ (800 mg loading dose, then 400 mg daily) IV., is an adequate alternative treatment for clinically stable, azole antifungal therapy-naïve patients with FCZ-susceptible clinical isolates (strong recommendation, moderatequality evidence) (Table 8, Annex 13) and Annex 14)1,43,206-208.
209. The consensus panel recommends in patients with abdominal sepsis, implementing a therapy de-escalation scheme (after 5-7 days) from an echinocandin to FCZ (400-800 mg daily) OA. or IV., if patients are clinically stable, apt for oral administration, azole antifungal therapy-naïve, and have FCZ-susceptible clinical isolates (strong recommendation, moderate-quality evidence) (Table 4, Annex 9 and 10) (Section: Candidemia/IC in Non-Neutropenic Patients)43,206.
210. The duration of antifungal therapy should depend on the adequate surgical control of the abdominal infective foci and patient’s clinical response (strong recommendation, low-quality evidence)209.
XLVII. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy in patients undergoing peritoneal dialysis and/or with secondary peritonitis?
Recommendation
211. The consensus panel recommends initiating EAFT in patients with Candida Spp. peritonitis or undergoing peritoneal dialysis (strong recommendation, moderatequality evidence).
212. The consensus panel recommends adequate percutaneous or surgical drainage to control the infective foci, and removing the peritoneal catheter for Candida Spp. peritonitis in patients undergoing peritoneal dialysis with intraabdominal abscesses (strong recommendation, moderate-quality evidence)208.
213. The consensus panel recommends initiating of antifungal therapy with an echinocandin (CAS [70 mg loading dose, then 50 mg daily], ANI [200 mg loading dose, then 100 mg daily], MIC [100 mg daily]), in patients with Candida Spp. peritonitis or undergoing peritoneal dialysis at risk of candidemia/IC (strong recommendation, moderatequality evidence) (Table 8, Annex 13 and Annex 14)43,72,206.
214. The consensus panel considers that there are no differences between echinocandins in the clinical setting of patients undergoing peritoneal dialysis and/or with Candida Spp. secondary peritonitis. The choice will depend on interactions with other drugs, liver failure, side effects and treatment costs (strong recommendation, moderate-quality evidence)11,74,75.
215. FCZ (800 mg loading dose, then 400 mg daily) IV., is an adequate alternative treatment for clinically stable, azole antifungal therapy-naïve patients with FCZ-susceptible clinical isolates (strong recommendation, moderatequality evidence) (Table 8, Annex 13 and Annex 14)43,206.
216. The consensus panel recommends in patients undergoing peritoneal dialysis and/or with secondary peritonitis, implementing a therapy de-escalation scheme (after 5-7 days) from an echinocandin to FCZ (400-800 mg daily) OA. or IV., if patients are clinically stable, apt for oral administration, azole antifungal therapy-naïve, and have FCZ-susceptible clinical isolates (strong recommendation, moderate-quality evidence) (Table 4, Annex 9 and 10) (Section: Candidemia/IC in NonNeutropenic Patients)1,72,208.
217. The consensus panel recommends removing the peritoneal dialysis catheter and targeted antifungal therapy for at least 2 weeks, in patients undergoing peritoneal dialysis and/or with secondary peritonitis at risk of candidemia/IC (strong recommendation, moderate-quality evidence)72,208-211.
Candida Spp. urinary tract infections
Doses in adult patients are established in the following recommendations. See Table 8 for doses in pediatric patients.
XLVIII. What is the diagnostic meaning of a Candida Spp. isolate from urine in asymptomatic patients?
Recommendation
218. Initiation of an antifungal therapy is not recommended in patients with asymptomatic candiduria or with minimal symptoms of candiduria (strong recommendation, high-quality evidence)10,212.
219. The consensus panel recommends eliminating existing predisposing factors such as indwelling bladder catheters in patients with asymptomatic candiduria, where possible (strong recommendation, high-quality evidence)10,212.
XLIX. What is the diagnostic meaning of a Candida Spp. isolate from urine in symptomatic patients? What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy?
Recommendation
220. The consensus panel recommends initiating antifungal therapy in patients diagnosed with symptomatic candiduria (strong recommendation, high-quality evidence)213,214.
221. The consensus panel recommends initiating antifungal therapy with FCZ (400 mg [6 mg/kg] daily) OA. or IV., for 2 weeks (strong recommendation, moderate-quality evidence) (Table 8, Annex 13 and 14)10,215.
222. The consensus panel recommends initiating single-dose antifungal therapy with AmB-D (0.3-1 mg/kg) IV. for clinical isolates suspected to be azole-resistant (weak recommendation, high-quality evidence) (Table 4, Annex 9)10,215.
223. The consensus panel recommends eliminating existing predisposing factors such as indwelling bladder catheters in patients with symptomatic candiduria, where possible (strong recommendation, high-quality evidence)10,215.
224. Irrigation of the urinary tract with AmB is not recommended in patients with symptomatic candiduria (strong recommendation, high-quality evidence)214.
L. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy in patients diagnosed with candidal pyelonephritis?
Recommendation
225. The consensus panel recommends initiating antifungal therapy with FCZ (400 mg [6 mg/kg] daily) OA. or IV., for 2 weeks, in patients with candidal pyelonephritis (strong recommendation, high-quality evidence) (Table 8, Annex 13 and Annex 14)10,213.
226. The consensus panel recommends initiating antifungal therapy with AmB-D (0.3-1 mg/kg) IV. or CAS (70 mg loading dose, then 50 mg daily) for 2 weeks, for clinical isolates suspected to be azole-resistant (strong recommendation, high-quality evidence) (Table 4, Annex 9)10,213.
LI. What is the recommendation for choosing the type of drug, dose, and duration of antifungal therapy in patients diagnosed with fungus ball?
Recommendation
227. The consensus panel recommends a surgical intervention and initiating antifungal therapy with FCZ (400 mg [6 mg/kg] daily) OA. or IV. for two weeks, after surgical removal of urinary mycetoma, in patients diagnosed with fungus ball (strong recommendation, moderatequality evidence) (Table 8, Annex 13 and Annex 14)10,216.
228. The consensus panel recommends initiating antifungal therapy with AmB (AmB-D [0.3-1 mg/kg], AmB-L [3-5 mg/kg daily]) for 2 weeks, for clinical isolates suspected to be azole-resistant (weak recommendation, highquality evidence) (Table 4, Annex 9) 10.
Candida Spp. respiratory tract infection
Doses in adult patients are established in the following recommendations. See Table 8 for doses in pediatric patients.
LII. What is the diagnostic meaning of a Candida Spp. isolate from upper and/or lower respiratory tract samples?
LIII. What is the use of performing cultures of respiratory tract samples for the initiation of antifungal therapy in patients with suspected IC?
LIV. Is antifungal therapy initiation recommended for Candida Spp. isolates from respiratory tract samples?
Recommendation
231. Initiation of targeted antifungal therapy is not recommended for Candida Spp. clinical isolates from respiratory tract samples, in the absence of a positive “Candida score” (strong recommendation, high-quality evidence) (Table 3) (Section: Diagnosis of Invasive Candidiasis [IC])10,219.
Prevention of Candida Spp. IFDs
LV. What special considerations should be taken into account in the pharmacological and non-pharmacological prevention of Candida Spp. IFDs?
Recommendation
232. The consensus panel considers that a daily bathing with 2% chlorhexidine reduces the incidence of candidemia/ IC, in patients older than two months of age and in adults and may be considered in high-risk patients (weak recommendation, moderate-quality evidence)220,221.
233. The consensus panel considers that bovine lactoferrin (100 mg daily) may be effective for preventing Candida Spp. IFDs, in neonate patients weighing < 1500 g (weak recommendation, moderate-quality evidence)222.
234. Hands sanitization and adherence to guidelines for the prevention of CVC related infections are recommended as preventive measures against Candida Spp. IFDs (strong recommendation, high-quality evidence)223,224.
235. A rational protocol for the management of antimicrobial agents and control of bacterial infections, is considered a useful preventive measure against Candida Spp. IFDs (strong recommendation, high-quality evidence)225,226.
236. Knowledge of the local epidemiology of each healthcare center, is considered a useful preventive measure against Candida Spp. IFDs (strong recommendation, high-quality evidence)225,226.
237. When outbreaks of Candida species considered “emerging” occur, isolating the patients, following-up all the cases and implementing the required measures to contain the outbreak, are considered as useful preventive measures against IFDs (strong recommendation, moderate-quality evidence)227,228.
238. The consensus panel considers that antifungal prophylaxis helps in reducing the prevalence of candidemia/IC in high-risk patients (strong recommendation, high-quality evidence) (Section: Antifungal Prophylaxis for candidemia/IC)229,230.
Epidemiology
Persons with fungal infections are generally chronically ill patients with worsened health status, severe immunosuppression levels and using different medical devices1-7. Candida Spp. IFDs account for 70-90% of all IFDs3 . Candidemia has a global prevalence of 19% and is the fourth cause of IFDs in critical patients, only surpassed by Pseudomonas Spp. (19.9%) and Staphylococcus aureus (20.5%) IFDs in this population of patients. It is considered the 7th-10th cause of IFDs in hospitalized patients2 .
The epidemiology of Candida Spp. infections has significantly changed over the last years, mostly because of medical-scientific advancements developed for the care of hospitalized patients1,2. IC/candidemia is thought to affect over 250,000 patients around the world and is the direct cause of over 50,000 deaths; the incidence of infectious disease ranges from 2 to 14 cases out of 100,000 inhabitants3-5, and in most of the regions, these rates have increased or remain stable. In initially high-incidence zones, it was observed that incidence decreased after the implementation of diagnostic and therapeutic management improvements3-5.
The epidemiological profile of Candida Spp. IFDs varies between regions and countries with differences in the distribution of species per geographical area, local epidemiological factors, prior exposure to antifungal agents or patient underlying conditions231-233. The current incidence of IC has remained consistent over the last years, or has slightly decreased in Australia, Canada, Europe, and the United States; however, its incidence in Latin America and the rest of the world is increasing233. In Australia, Canada, Europe and Latin America the incidence of candidemia is significantly lower than in the United States, where 6-10 cases out of 100,000 inhabitants were reported6,7. In contrast, Europe reports incidence rates of 1.4-5.7 cases out of 100,000 inhabitants,except in Denmark and Spain where IC rates are higher. Most of the Nordic countries have reported IC/candidemia rates of 1.4-5.7 cases out of 100,000 inhabitants; the incidence in Australia (1.8 cases out of 100,000 inhabitants) and Canada (2.9 cases out of 100,000 inhabitants) is similar to that of Europe233-236.
Even though the epidemiology of candidemia in Latin America has not been comprehensively studied, a total incidence ranging from 1.18 to 2.49 cases out of 1000 hospital admissions has been reported76,237. Although wide variations were found among Latin American countries (0.33 cases in Chile vs 1.96 cases out of 1000 hospital admissions in Argentina and Colombia), the mean incidence is higher than that reported in the United States (0.28-0.96 cases out of 1000 hospital admissions) or Europe (0.2-0.38 cases out of 1000 hospital admissions)233. In Colombia, Cortes et al. reported a prevalence of candidemia in ICUs of 1.4-5.2%, with infection rates of 2.3 cases out of 1000 hospitalization days238,239. The Germen group reported that from 22,630 blood cultures taken between 2010 and 2011, 728 (3.3%) isolates corresponded to Candida Spp.; C. albicans was the most common (38.7%, n= 335), followed by C. parapsilosis (20.9%, n= 192), C. tropicalis (16.1%, n= 148) and C. glabrata (12.2%, n=112); susceptibility to fluconazole (FCZ) corresponded to 94.3%, 80.3%, 97.5% and 91.5%, respectively240. The CIDEIM Center et al. reported that from 2010 to 2013, Candida Spp. isolates from blood cultures from patients in an ICU held the third place, and the most common species were Candida non-albicans241. The GREBO group2383-242. reported that, in general, Candida Spp. isolates held the sixth place in prevalence with a trend towards Candida non-albicans isolates; in addition, they established an overall mortality rate of 36%, associated with the age and existence of septic shock at the moment of the diagnosis of candidemia238
There is no clear reason as to why the incidence of IC/candidemia is higher in Latin America, the United States, Denmark or Spain, but the heterogenicity of the methodology and the size of the sample reported, the diversity of the methodology of the investigations, the distribution of age, and risk factors of the populations studied may have contributed to those findings. In addition, in the Latin American setting the following aspects should also be considered: (1) differences in the available healthcare resources and training programs, (2) difficulties in the implementation of infection control programs in hospitals in developing countries, and (3) the limited number of healthcare workers available for taking care of this type of infections233.
Distribution of species and resistance to antifungal agents
In general, Candida Spp. IFDs are conditions associated with medical progress and they are one of the main causes of morbidity and mortality in the health setting. It has been observed that only five species (Candida albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis, andCandida krusei) account for more than 90% of the isolates, and each species has a unique potential virulence, antifungal susceptibility, and epidemiology233,243 C. parapsilosis and C. krusei are less virulent than C. albicans, C. tropicalis and C. glabrata; this variation is reflected in the low mortality rate of patients with C. parapsilosis candidemia, and the low frequency of C. krusei invasive infection, except for patients with serious immunodeficiency and with a history of prior exposure to azoles. Despite its low virulence, C. parapsilosis may thrive in certain clinical niches, because of its ability to adhere to medical devices and its propensity for colonizing the human skin, less frequent characteristics in other species such as Candida dubliniensis, Candida lusitaniae, Candida kefyr and Candida guilliermondii, which are associated with reduced susceptibility patterns in specific populations233.
C. albicans is still the most common species worldwide (38%-70% of the cases), even though its incidence is decreasing because of the increased incidence of C. non-albicans species3,237,238,243; C. glabrata has emerged as an important pathogen in Northern Europe, the Unites States and Canada, while C. parapsilosis is more common in Southern Europe, Asia, and Latin America232. In the United States, C. albicans isolates have decreased, and C. glabrata isolates have increased, becoming the second most common cause of candidemia (after C. albicans) in 20%-26% of the cases. In Europe, C. albicans is still the first etiological agent associated with candidemia and C. non-albicans species account for 43.6% of IFDs, but are considered the most common species in hematological patients9,232.
In Latin America, C. albicans also hold the first place (37.6%) among isolates and, contrary to that reported in Europe and the United States, C. glabrata isolates are still a few (6.3%), and C. parapsilosis (26.5%) and C. tropicalis (17.6%) are considered the most common species after C. albicans76. In Colombia, the distribution of species is similar to that reported in Latin America76,240,242, isolates from blood cultures of patients in ICU are associated with C. albicans (56%), C. tropicalis (17.3%) and C. parapsilosis (16%)238,239 and increasing incidence of C. non-albicans has been reported241.
Recently, Candida auris, a cryptic species uncommon in most hospitals around the world, has appeared as an emerging species and a global threat capable of developing resistance to multiple antifungals and with great potential for nosocomial transmission, whose mortality percentages can be between 27 and 60%, although the burden of infection may be underestimated, since conventional automated methods fail to correctly identify the species, so molecular or protenomic methods are necessary; the appearance of candidemia by C. auris probably depends on the conditions of the patients, the control of the source of infection and the initiation of an adequate antifungal therapy243. To date, colombian reports reveal widespread environmental contamination and colonization among patients and health workers, where unlike different reports worldwide, the clinical and environmental isolates of C. auris present a good susceptibility to the echinocandins,with a variable resistance to Amphotericin B (AmB)242-244.
Risk factors associated with candidemia/IC
Increasing incidence of candidemia is associated with the higher complexity of surgical procedures, populations at higher risk of infection and changes in the demographic characteristics of patients. The clinical manifestation of candidemia varies depending on: (1) age (infants < 1 year of age, and adults > 65 years of age: 16 to 36 cases out of 100,000 inhabitants), (2) type of patient (in hemato-oncological patients [71 cases out of 100,000 inhabitants] and in diabetic patients [28 cases out of 100,000 inhabitants]), (3) presence of central vascular catheters (CVC), (4) surgical history (particularly in abdominal surgery with anastomotic leaks), and (5) administration of antimicrobial agents (Table 2)233.
Cancer is a common underlying disease in patients with candidemia, but different in its manifestation, depending on its oncologic diagnosis. In patients with hematological malignancies, chemotherapy and subsequent neutropenia, digestive tract mucositis, and treatment with corticosteroids are risk factors for IC/candidemia. In patients with solid tumors, candidemia is associated with surgical complications, admission to ICU, mechanical ventilation, overeating and the presence of CVC233. Survival of critically ill patients has resulted in increased use of invasive and therapeutic procedures which have favored increased rates of mortality associated with fungal multicolonization, the virulence of the species involved, inadequate antifungal therapy or delayed treatment initiation, the level of patient’s involvement and inadequate control of the infective foci36,245,246.
Methodology
Members of the panel
For developing this consensus, a multi-disciplinary Panel of 18 specialists (pediatrics, internal medicine, infectious diseases, mycology, and epidemiology) from the national territory, experts in the treatment of adult, pediatric and neonate patients with Invasive Mycoses (IM), ACIN-members, was convened. All members of the Panel were selected based on their experience in the research, diagnosis, treatment, and follow-up of IFDs.
Process overview
The work plan of the consensus was developed following the RAND/UCLA method, which is based on scientific evidence and collective judgment of an Expert Panel248. A series of questions were developed, taking into account critical factors conditioning decision making in patients with Candida Spp. infectious disease. Each member of the Panel was assigned to review recent literature on at least one topic of the consensus in order to evaluate the evidence, establish the strength of the recommendations and develop written evidence to support such recommendations. The Panel reviewed and discussed all the recommendations, their strength and the quality of evidence. Discrepancies associated with the presentation of evidence were collectively discussed and resolved, and the final recommendations represent the consensual opinion of the Panel. All the sections were collectively reviewed by the Panel for the final version of the consensus.
Review of evidence
To evaluate the quality of evidence and strength of recommendations, the modified GRADE approach was used9,248whereby each recommendation is assigned a separated classification for the underlying quality of the evidence supporting the recommendation and for the strength with which the recommendation is made. The following levels of evidence were established: LOW (III): results can substantially change over time; MODERATE (II): results can change over time, but not substantially; HIGH (I): the likelihood that results could change is low. The strength of the recommendation (WEAK or STRONG) was evaluated taking into account the benefitrisk balance, quality of evidence, patient’s values and preferences, and cost or use of resources249. The quality of evidence was evaluated by the AGREE II instrument250-253, including selected guidelines with a mean score of evaluated domains > 60%, as substrates for the consensus (Annex 1 and 2)254. With the selected guidelines and consensus, a document issuing recommendations for the questions made was drafted; the Panel met in person once and attended several videoconferences for 10 months, where recommendations were individually pointed out via the modified Delphi technique33, with two rounds of voting (secret and open). A consensus was reached by over 75% agreement of the Expert Panel for each recommendation (Annex 1 and 2).
Systematic reviews
A bibliographic search of clinical practice guidelines for Candida Spp. infections and guidelines on Candidiasis including recommendations for different target population groups of the consensus (adult, pediatric and neonatal) was conducted, using sources from compilation bodies (NGC, National Guideline Clearinghouse, Guideline International Network), clinical practices guidelines producers (New Zealand Guidelines Group, National Institute for Clinical Excellence, Scottish Intercollegiate Network), Iberoamerican clinical practice guidelines and general databases (Pubmed, Medline, EMBASE). The following terms were used MESH: Candidemia, Candidiasis, Invasive Candidiasis, antifungal prophylaxis, prophylaxis, diagnosis, adult patient, pediatric patient, neonate patient, nonneutropenic patient, neutropenic patient, critical patient, recommendations, antifungal therapy, consensus, consensus guidelines, amphotericin B, azoles, echinocandins, 5-fluorocytosine, fungal diagnosis, antifungal agents, biomarkers, development of guidelines, management, Latin America, antifungal stewardship, antifungal resistance, hematological malignancy, rapid diagnostic, transplantation, leukemia, cancer. Only guidelines published after 2012 and specific topic reviews from 2010 onwards were taken into account.
Table 1 Rating scale of the quality of evidence and the strength of recommendations8 .

Adapted from: Andrews JC et al8.
Table 2 Predisposing factors and populations at risk of candidemia/IC247.

Adapted from: Pemán J et al247.
ICU: Intensive care unit; CVC: Central venous catheter.
Table 3 Description of clinical indexes used to determine the risk of developing IC17.

Adapted from: Garnacho-Montero J et al17.
*S/Sp: Sensitivity/Specificity; PPC/NPV: Positive predictive value/Negative predictive value. a Heavy colonization: Candida spp. growth ≥105 UFC/ml NMC Rule: (1.537 × broad-spectrum antibioticsb) + (0.873 × CVCb) + (0.922 × PNb) + (0.402 × corticosteroidsc ) + (0.879 × abdominal surgery) + (0.039 × Mean length of hospital stay before admission to ICU) bDays 1 to 3 in ICU c Days 7 to 3 in ICU
Table 4 Common yeasts susceptibility to antifungal drugs11,23.

Adapted from: Arendrup et al.; Pappas et al11,23,243.
a C. parapsilosis group comprises three species: C. metapsilosis, C. orthopsilosis and C. parapsilosis. bThe susceptibility of C. glabrata depends on the geographic area, fluconazole is not recommended. c ~ 50% and ~ 30% of C. glabrata and C. krusei isolates, respectively, are itraconazole-resistant. dSusceptible, even though available clinical data are limited. e 20% of the isolates are amphotericin B-resistant f This species resistance cutoff point has not been defined.
+https://www.cdc.gov/fungal/diseases/candidiasis/c-auris-treatment.html.
*Anidulafungin, caspofungin and micafungin.
S: Susceptible; I: Intermediate; R: Resistant; DDS: Dose-dependent susceptible.
Table 5 Risk factors and antifungal prophylaxis against IC in SOT38.

Adapted from: Aguado JM et al38.
FCZ: Fluconazole; AmB-D: Amphotericin B deoxycholate; AmB-L: Liposomal amphotericin B; CAS: Caspofungin; OA: Oral administration; CMV: Cytomegalovirus.
Table 6 Recommendations for the administration of antifungal prophylaxis against Candida spp. to non-neutropenic adult patients in the ICU43.
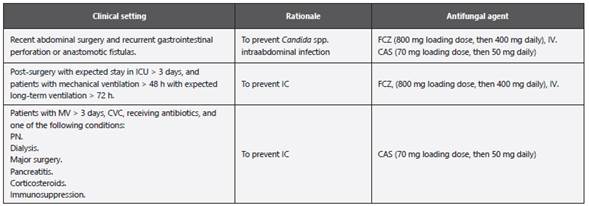
Adapted from: Cornely OA et al43.
ICU: Intensive care unit; MV: Mechanical ventilation; CVC: Central venous catheter; PN: Parenteral nutrition; IC: Invasive candidiasis; FCZ: Fluconazole; CAS: Caspofungin; IV.: Intravenous administration.
Table 7 Additional follow-up tests for adult patients following the diagnosis of candidemia10,14

Adapted from: Nucci M et al.; Pappas PG et al10,14. CVC: Central venous catheter.
Table 8 ADME and doses of systemic antifungal drugs29,77,78,80,299,300.

Adapted from: Mensa-Pueyo J. et al.; Gilbert DN et al.; Ruiz-Camps I. et al.; Cuenca-Estrella M.; Lewis RE.; Bellmann R et al29,77,78,80,299,300.
A: Administration; D: Distribution; M: Metabolism; E: Excretion; AmB-D: Amphotericin B deoxycholate; AmB-L: Liposomal amphotericin B; AmB-CL: Amphotericin B lipid complex; GF: Glomerular filtration; IV.: Intravenous administration; OA: Oral administration; d: Days; h: Hours; g: Grams; mg: Milligrams; kg: Kilograms; HD: Hemodialysis; CAPD: Continuous ambulatory peritoneal dialysis; CRRT: Continuous renal replacement therapy; CNS: Central nervous system.
Table 9 Risk factors for the development of Candida spp. IFDs in pediatric population330.

Adapted from: Figueras C et al330.
CVC: Central venous catheter; CNS: Central nervous system; PN: Parenteral nutrition; MV: Mechanical ventilation.
Table 10 Risk factors for neonatal candidiasis98.

Adapted from: Kaufman DA98.
CVC: Central venous catheter; PN: Parenteral nutrition.
Material suplementario disponible en pdf: v23n3a12.pdf