INTRODUCTION
The Big-eared Brown Bats of the genus Histiotus are endemic to South America, occurring along the Andean chains in a variety of mountainous ecosystems, from Venezuela and Colombia to the most southern continental lands of Argentina and Chile, the coastal Atlantic Forest of eastern Brazil, and the semiarid regions of Argentina and Brazil (Handley and Gardner 2008, Feijó et al. 2015, Moratelli et al. 2019). Histiotus are easily differentiated from other South American Vespertilionids by their very long pinnae and large tympanic bullae (Handley and Gardner 2008). Recently, molecular phylogenetic analyses found that Histiotus was nested within the clade of the New World Eptesicus (Hoofer and Van Den Bussche 2003, Roehrs et al. 2010, Amador et al. 2018). However, the relationships among worldwide Eptesicus remain unresolved and no taxonomic decisions have been taken. Despite that, based on the unique morphology within the South American vespertilionid bats, Histiotus has been recognized as a valid genus in recent taxonomic treatments (Simmons 2005, Handley and Gardner 2008, Burgin et al. 2018, Moratelli et al. 2019).
The alpha taxonomy of Histiotus has been also controversial and the species limits unclear, due to the similar interspecific skull morphology, few specimens available in collections, and scarce available molecular data with limited taxonomic and geographic representativeness (Feijó et al. 2015, Giménez et al. 2019). The number of recognized species has varied between four and eight (Handley and Gardner 2008, Giménez et al. 2019, Moratelli et al. 2019). For example, Simmons (2005) recognized seven species: H. alienusThomas, 1916, H. humboldti Handley, 1996, H. laephotis Thomas, 1916, H. macrotus (Poeppig, 1835), H. magel-lanicus Philippi, 1866, H. velatus (I. Geoffroy, 1824), and H. montanus (Philippi and Landbeck, 1861), the latter with two additional subspecies, H. m. colombiae Thomas, 1928, and H. m. inambarus Anthony, 1920. In contrast, Handley and Gardner (2008) considered H. alienus, H. magellanicus, H. laephotis as subspecies of H. montanus. Recently, Moratelli et al. (2019) recognized eight species adding H. diaphanopterus Feijó, Da Rocha and Althoff, 2015 to the seven species recognized by Simmons (2005).
Contributions on the taxonomy and distribution of Histiotus are few and mostly dedicated to southern South American forms. For instance, Feijó et al. (2015) described H. diaphanopterus from northeastern Brazil, and Giménez et al. (2019) showed molecular evidence of specific level differentiation between H. montanus and H. magellanicus in Argentina and Chile. In contrast, the northern forms, H. humboldti and H. m. colombiae, distributed along the Andes of Venezuela, Colombia, and Ecuador (Handley and Gardner 2008, Moratelli et al. 2019), remain poorly studied (Rodríguez-Posada 2010).
In a taxonomic account on Histiotus of the Cordillera Central of the Andes of Colombia - one of the three roughly parallel chains of the Andes of the country (namely Cordilleras Occidental, Central, and Oriental), extending northeastward almost to the Caribbean Region - Rodríguez-Posada (2010) raised two important issues on the taxonomy of the Colombian Histiotus. First, the author considers that due to the wide distribution of H. montanus and the recognition of the validity of three of its historical subspecies as full species, H. m. colombiae should be considered as a full species too. Second, the author notices morphological differences between specimens identified as H. montanus (assigned to H. m. colombiae) from the Central and Oriental cordilleras that suggest possible cryptic diversity within Histiotus. To evaluate both issues, we reviewed the alpha taxonomy and distribution of His-tiotus in Colombia using morphological, morphometric, genetic, and acoustic evidence.
MATERIALS AND METHODS
Morphological analyses
We studied 103 specimens belonging to eight proposed taxa of Histiotus according to Simmons (2005) and Moratelli et al. (2019): H. montanus montanus, H. m. colombiae, H. m. inambarus, H. diaphanopterus, H. humboldti, H. laephotis, H. macrotus, and H. velatus. We also included specimens from two independent undescribed taxa, both from Peru. One of them is based on one specimen from Cajamarca housed at the American Museum of Natural History (AMNH 268090) that could not be assigned to any Histiotus species known to date. The second has been mentioned in the literature as Histiotus sp. by Giménez et al. (2019) based on two specimens (AMNH 278521, 278524) from Piura (Supplementary material, Appendix 1). We recorded the sex, the locality, and five external measurements from the specimen's labels (in mm). Thirteen craniodental and mandibular measurements were taken from specimens with calipers accurate to the nearest 0.01 mm. The eighteen standard measurements, including external, craniodental and mandibular, are based on Handley (1996), Moratelli et al. (2013), and Feijó et al. (2015). These measurements are described in appendix 2 of the supplementary material: total length (TL), tail length (TaL), hindfoot length (HFL), ear length (Ear), forearm length (FA), greatest length of skull (GLS), condylo-incisive length (CI), zygomatic breadth (ZB), postorbital breadth (PO), braincase breadth (BCB), mastoid breadth (MB), braincase height (BCH), length of the maxillary tooth row (MTR), palatal length (PAL), post-palatal length (PPAL), breadth across upper molars (M-M), breadth across the canines (C-C), mandibular tooth row (Dent-L).
To evaluate morphometric variation within Histiotus from Colombia, we performed a Principal Component Analysis with the thirteen cranial measurements using the statistical package PAST version 4 (Hammer et al. 2001). We used the covariance matrix to preserve the information about the relative scale among variables and all data were transformed log10. Due to the completeness of our dataset, only 60 adult specimens (Appendix 1 in supplementary material) with all the variables representing eight taxa (H. humboldti, H. laephotis, H. velatus, H. macrotus, H. montanus montanus, and H. montanus colombiae) were included in our morphometric analyses. For H. m. colombi-ae we included samples from both the cordilleras Oriental and Central of Colombia and from northern Ecuador. To find discrete characters supporting morphometric groups we also explored the diagnostic external and craniodental traits available in the literature for all studied specimens.
Molecular methods
We extracted whole genomic DNA from two individuals of H. montanus colombiae and four individuals of H. hum-boldti from the Cordillera Oriental of Colombia, using standard phenol-chloroform methods (Sambrook et al. 1989) on muscle tissues preserved in 96 % ethanol. Unfortunately, we could not access to tissues of specimens identified as H. montanus colombiae from the Cordillera Central. Preservation in formalin of ICN specimens refrain us from successful DNA amplification. Amplification of cytochrome-b (Cyt b) was performed using primers glo7L and glo6H (Hoffmann and Baker 2001). A partial sequence between 900 and 1100 base pairs of all individuals was amplified and sequenced following Porter et al. (2007), although we increased the annealing temperature from 45-48 °C to 50.5 °C. We carried out purification and sequencing of both strands with the amplification primers on an ABI 3500 sequencer (Applied Biosystems, Waltham, MA, USA) at the Servicio de Secuenciación y Análisis Molecular SSiGMol at the Universidad Nacional de Colombia, Colombia, Bogotá. We submitted genetic sequences to Gen-Bank under accession numbers presented in Table 1 in the supplementary material. Sequences were manually checked and aligned using BioEdit 7.2.6 software (Hall 1999). We checked for discontinuities and stop codons in each sequence using the ExPASy translate web tool (https://web.expasy.org/translate/).
Table 1 Craniodental measurements (mm), including the mean, the standard deviation, the observed range, and the sample size of H. humboldti, H. cadenai, H. colombiae, and H. montanus. Measurement acronyms are in the main text.

We retrieved two additional sequences from BoldSys-tems of H. m. colombiae provided by Instituto de Recursos Biológicos Alexander von Humboldt, and 17 complete Cyt b gene (1140 bp) sequences from GenBank representing four species of Histiotus (H. m. montanus, H. magel-lanicus, H. macrotus, and H. sp. from Peru), and the out-groups Eptesicus fuscus (Palisot de Beauvois, 1796) and Myotis riparius Handley, 1960, to be used in phylogenet-ic analyses and genetic distance comparisons (accession numbers available in Table 1 in supplementary material). The complete data set consisted of 26 sequences. We inferred a phylogenetic tree using Maximum Likelihood analyses in RAxML 1.5 beta software (Stamatakis 2014), and Bayesian Inference analyses using MrBayes v.3.1 software (Ronquist and Huelsenbeck 2003), running 10 x 106 generations with one cold and three incrementally heated Markov chains, random starting trees for each chain, and trees sampled every 100 000 generations. We discarded 20 % of the resulting trees as burn-in, and 85 % of the trees were used for generating a 50 % majority-rule consensus. Statistical support for resulting phylogenies was measured in bootstrap support values (of 500 iterations) and Bayes-ian posterior probabilities. Genetic distance values were estimated using the p-distance method.
Acoustic characterization
We recorded echolocation calls of H. montanus colombiae from the Cordillera Oriental using an ultrasound bat-detector (Anabat Swift, Titley Scientific) with a sampling rate of 500 kHz. The bat detector was placed at a height of two meters above ground level in an open space, close to a roost in an abandoned rural house roof (Cundinamarca: Guachetá, Gachetá Alto: 5°27'39.47"N; 73°39'44.57"W; 2893 m). We collected four voucher specimens to confirm the species identification and deposited them at the Colección de Mamíferos Alberto Cadena, Instituto de Ciencias Naturales - ICN collections (ICN 24845-24848). We analyzed only the recordings with at least five pulses of good signal to noise ratio. Sequences of echolocation calls were displayed simultaneously as spectrograms and oscillograms using the software Raven Pro 1.6.1 (Center for Conservation Bioacoustics 2019). Spectrograms were made of consecutive fast Fourier transformations with an 85 % overlap and visualized on a Hamming type window. For each pulse, we manually measured the following parameters: 1) duration (time between start and end of a call, measured in milliseconds); 2) peak frequency (frequency in kHertz corresponding with maximal intensity in the power spectrum), 3) start frequency, 4) end frequency, and 5) interpulse interval (measured from the beginning of a call to the start of next call). We measured these variables to compare them with the data from H. montanus montanus from Chile reported by Ossa et al. (2015). All the values of acoustic parameters are given as mean ± 1 SD, since the data sets were not normally distributed, we used nonparametric statistics (Kolmogorov-Smirnov test). We carried out the tests using the software PAST version 4 (Hammer et al. 2001).
RESULTS
Morphological traits
Based on exclusive combinations of discrete characters, for Colombia, we identified three morphological groups of Histiotus including H. humboldti, H. m. colombiae from the Cordillera Oriental and H. m. colombiae from the Cordillera Central and northern Ecuador; the latter was considered as a new species described below (see comparisons below and Table 2 in supplementary material). In addition, there is morphological evidence to differentiate the nominal H. montanus from southern South America from the three morphological forms found in Colombia (see description and comparisons below and Table 2 of the supplementary material).
The PCA analysis of the morphometric data including specimens from all South America shows that the first two principal components (PC) accounted for 78.8 % of the total variance (PC1 = 70.9 %, PC2 = 7.9 %). The length of the maxillary toothrow (MTR), the palatal length (PAL), the breadth across upper molars (M-M), and breadth across canines (C-C) were related with PC1 and the postorbital breadth (PO) with PC2.
The projection of the first two components of the PCA (Fig. 1) showed five morphological groups. H. humboldti is observed in the negative extreme of the PC1. H. m. montanus from southern South America and H. m. colombiae are separated in the morpho-space in two distinct discrete groups in the positive axis of the PC1. Specimens identified as the new species from Colombia and Ecuador appeared also separated from H. montanus colombiae, although have a small overlapping. Other morphological distinctive taxa such as H. velatus are located at the center of the PC1, and two specimens of H. lapheotis and one specimen of H. diaphanopterus are isolated in the negative extreme of the PC2 (center of the PC1). One specimen (AMNH 268090) identified as Histiotus sp. is immersed within the new species of Histiotus from Colombia and Ecuador. A second specimen identified as Histiotus sp. (AMNH 278521) is isolated between H. velatus and the H. montanus group. Finally, an individual of H. macrotus is immersed in an extreme of the H. montanus colombiae group.

Figure 1 Scatter plot of the first and second principal component scores from the principal component analysis. The analysis was based on 13 crania and mandibular measurements from seven species of Histiotus including H. humboldti, H. laephotis, H. velatus, H. laephotis, Histiotus sp. n. (described here as H. cadenai)., H. colombiae, H. macrotus, and H. montanus; and two H. sp. which could not be assigned to any Histiotus species. H. sp.*: AMNH 278521 from Peru, Piura. H. sp.**: AMNH 268090 from Peru, Cajamarca.
Molecular analyses
Sequence alignment of the 23 cytochrome-b sequences of Histiotus was unequivocal and without internal stop codons. Four sequences of H. m. colombiae from the Cordillera Oriental of Colombia were recovered as a mono-phyletic group with strong support (Bootstrap = 100, Posterior probability = 1, Fig. 2) and separated for H. m. montanus from Argentina, the latter forming a clade with H. macrotus.

Figure 2 Combined tree from Bayesian and maximum likelihood analysis based on 23 cytochrome-b gene sequences of six species of Histiotus. Numbers in parentheses designate individual specimens listed in Appendix 3. Branch support is given for supports above 0.90 in the Bayesian posterior probability and over 75 in bootstrap support values (percentage of 500 iterations) for maximum likelihood.
The mean genetic distances between H. m. colombiae from the Cordillera Oriental and other species of Histiotus range between 11.26 % and 12.75 %. Histiotus m. colombiae differs about 12.71 % from H. m. montanus from southern South America. Genetic distances from other species range from 0.18 % between H. m. montanus and H. macrotus, and 11.96 % between H. m. montanus and H. magellanicus. The intraspecific genetic distance value within H. m. colombiae from Cordillera Oriental was 1 % between four sequenced specimens (Table 3 in supplementary material).
Acoustic results
We obtained a total of seven wave files from H. m. colombiae from individuals flying in the surroundings of their roost. The echolocation calls from H. m. colombiae displays the downward frequency-modulated sweep design. Histiotus m. colombiae emitted search calls of a single harmonic. Calls are characterized by durations shorter than 6 ms emitted at intervals of 175.23 ± 54.5 ms (Fig. 3). The comparisons with H. m. montanus showed that although both species have a quasi-constant frequency component, the results from Kolmogorov-Smirnov test indicated significant differences for each one of the parameters (P < 0.005, Fig. 3). H. m. colombiae presented lower start (40.96 ± 4.0; N= 100 pulses), end (20.36 ± 0.8; N= 100 pulses) and peak frequencies (26.12 ± 1.7; N= 100 pulses) than H. m. montanus (start frequency (46.26 ± 4.4); end frequency (25.47 ± 1.9) and peak frequency (32.30 ± 1.8) N=90 pulses), and also displayed larger duration pulses (5.01 ± 1.3; N = 100 pulses) respect to H. m. montanus (3.10 ± 1.1; N=90 pulses; see Table 4 in supplementary material).
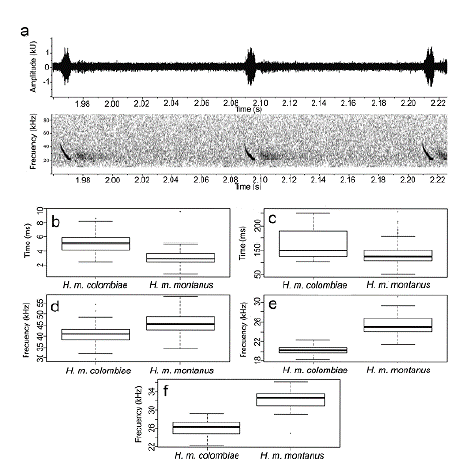
Figure 3 Characteristic of echolocation call of Histiotus m. colombiae and differences in call parameters with H. m. montanus. a. Oscillogram (above) and spectrogram (below) of the echolocation calls emitted by H. m. colombiae. Boxplots for each variable between H. m. colombiae from Colombia and H. m. montanus from Chile, b. Duration, c. Inter-pulse interval, d. Start frequency, e. End frequency, f. Peak frequency, Data of H. m. montanus were provided by Gonzalo Ossa.
Taxonomic remarks
We recognize the bats previously identified as H. montanus colombiae from the Cordillera Central of Colombia and northern Ecuador as a new species based on a unique combination of morphological traits (Table 2 in supplementary material) that did not match those of any known species of Histiotus. Additionally, we suggest the validity at species level (and discard clinal or subspecific variation) of H. colombiae based on a set of morphological traits (Table 2 in supplementary material), the wide genetic distance in the Cyt b gene (around 12.71 %; Table 3 in supplementary material) with the nominal H. montanus and the differences in the acoustical parameters between them. We conclude that the nominal H. montanus is distributed in southern South America in Argentina, Chile, and Uruguay.
SYSTEMATICS
CLASS MAMMALIA
ORDER CHIROPTERA
Family Vespertilionidae Gray, 1821
Genus Histiotus I. Geoffroy Saint-Hilaire, 1824
Histiotus cadenai Rodríguez-Posada, Ramírez-Chaves and
Morales-Martínez, new species
Cadena's Long-Eared bat.
Figs. 4-5b, f.
Histiotus montanus:Bejarano-Bonilla et al. (2007): 300, Rodríguez-Posada (2010): 176, not Vespertilio montanus Philippi and Landbeck, 1861.
Holotype. COLOMBIA. Caldas: $ fixed in formalin, preserved in alcohol with the skull removed and cleaned, Manizales, vereda el Desquite, corregimiento 7, Reserva Hidrográfica Río Blanco, finca Martinica, 5° 4'9.40"N, 75°22'38.28"W, 3500 m a.s.l., 6 Feb 2004, col: M. E. Rodríguez-Posada/ field number MRP 171/ICN 16980 (ICN). The external and cranial measurements of the holotype are: TL: 110, TaL: 50, HFL:12, Ear: 36, FA:48, Weight: 11.25, GLS: 17.45, CIL: 16.98, ZB: 10.73, PO: 4.68, BCB: 8.14, MB: 8.98, BCH: 5.99, MTR: 6.57, PPAL: 7.07, PAL: 7.76, M-M: 7.2, C-C: 4.88, Dent-L: 12.17.
Paratypes. COLOMBIA. Caldas: ♀ fixed in formalin, preserved in alcohol with the skull removed and cleaned, Manizales, vereda el Desquite, corregimiento 7, Reserva Hidrográfica Río Blanco, finca Martinica, 5° 4’9.40”N, 75°22’38.28”W, 3500 m a.s.l., 6 Feb 2004, col: M. E. Rodríguez-Posada /field number MRP 172/ICN 16981 (ICN). Quindío: ♂ skin and cleaned skull, Génova, predio Juntas, 04°08’N, 75°44’W, 3400 m a.s.l., 3 Dec 2004, col: P. X. Roa-A/ field number 20/ Universidad del Valle - UV 13274 (UV). Risaralda: ♀ skin and cleaned skull, Laguna del Otún, bosque del Oso, 3 km N, 1 km W, 4°46’N, 75°24’W, 3560 m a.s.l., 20 Aug 1980, col: M.S. Alberico/ field number 751/ UV 2524 (UV). Valle del Cauca: ♀ skin and cleaned skull, La Florida, Hacienda El Sinaí, 03°19’N, 76°11’W, 2900 m a.s.l., 21 Mar 1993, col: M.S. Alberico/ field number 2235 / UV 11031 (UV). ECUADOR. Pichincha: Sex unknown and ♂ skins and cleaned skulls, Piedra Blanca, ladera norte del volcán Rumiñahui, 0°34’S, 78°30’W, 4000 m a.s.l., 14 and 24 Oct 1992, col: G. Zapata et al./ No field number/ Pontificia Universidad Católica de Ecuador - QCAZ 690 and QCAZ 691 (QCAZ). Tungurahua: ♀ skin and cleaned skull, Patate, San Francisco, East of Ambato, 01°18’S, 78°30’W, 2438 m a.s.l., 15 Jan 1924, col: G. H. H. Tate / 2703/ AMNH 67648 (AMNH). Napo: ♀ skin and cleaned skull, El Chaco, Oyacachi, 5 km al este, confluencia del río Oyacachi con el río Chalpi, 00º25’S, 78º00’W, 2550 m a.s.l., 18 Feb 2007, col: P. Jarrín/ 18 Feb 1997/ QCAZ 2303 (QCAZ). The external and cranial measurements of the paratypes are in the Table 5 in supplementary material.
Other specimens. COLOMBIA. Tolima: ♂ preserved in alcohol with the skull removed and cleaned, Ibague, vereda La Cueva, 4°37'N, 75°19'W., 3550 m a.s.l., 2 Aug 2002, No collector data/ Colección Zoológica Universidad del Tolima - CZUT 259 (CZUT). A ♂ preserved in alcohol, Anzoategui, vereda El Palomar, 04°36'S, 75°11'W, 3297 m a.s.l., 9 Jul 2007, No collector data/ CZUT 674 (CZUT).
Distribution: Histiotus cadenai sp. n. is currently known to occur in 10 localities (Appendix 1 in Supplementary material) along the northern Andes in a latitudinal range from 01°18'S to 05°04'N. In Colombia, the species has been recorded in six localities in the Cordillera Central (departments of Caldas, Quindío, Risaralda, Tolima, and Valle del Cauca). In Ecuador, the species has been recorded in four localities of the northern portion of the Ecuadorean Andes (provinces of Napo, Pichincha, and Tungurahua). The known altitudinal distribution encompasses a gradient between 2550 to 4000 m a.s.l, including highland ecosystems such as Paramo, Andean montane forest, and anthropized areas. The distance between the extreme localities is approximately 797 km.
Etymology. The species epithet honors the Colombian pioneering mammalogist Alberto Cadena-García. He trained several generations of researchers currently working around the world on topics such as biology and conservation of mammals, continuing his legacy for new generations of biologists.
Diagnosis. This species is distinguished from all other species of the genus Histiotus by the following combination of characters: a medium-sized bat (total length: 102116 mm; forearm length: 45-50 mm; greatest length of skull: 17.12-18.13 mm), with long and silky dorsal fur light brown, ventral coloration is yellowish-brown. Ears are very long (length: 31-36 mm) and not connected by a band. Ears and patagium dark brown, darker than fur coloration. Pinnae are triangular with rounded points and a notch on the outer edge near the tip. The tragus is ensiform, with a sword-like shape with parallel edges and an acute tip. Calcar is well developed and keeled without lappet. The skull is robust, has a globular braincase with a continuous slope in lateral view, but the posterior border is rounded. Sagittal crest present but form a triangular plate of bone at the intersection with the nuchal crests. Nuchal crests are well developed. Paraoccipital process well developed wide and blunt. The anterior region of the braincase has the parietals straight and highly convergent, forming an abrupt angle with the frontal borders in a conspicuous waist shape towards the postorbital constriction. The supraorbital region is swollen without marked postorbital ridges. The upper toothrow is straight. The position of I2 is lateral to I1. Upper premolar without an anterior projection. M1 has a well-developed but blunt protocone. The angular process has a laminar shape, and it is longer than the condylar process, projected outward, and outside of the condylar process plane. The anterior edge of the coronoid process is straight and forms a 90° angle with the dentary.
Description. Histiotus cadenai is a medium-sized bat (total length: 102-116 mm; forearm length: 45-50 mm; greatest length of skull: 17.12-18.13 mm). The dorsal fur is long, silky, and light brown with a brown base (3/4), and light brown tips (1/4). Ventral coloration is yellowish-brown with a dark brown base (2/3) and creamy yellowish tips (1/3). The ears are very long (31-36 mm) and not connected by a band. Pinnae are deltate (equilateral triangle form) with rounded points and a notch on the outer edge near the tip. The tragus is ensiform with parallel edges and an acute tip, the edge is crenulated, but it appears smooth without magnification. Ears and patagia are dark brown, darker than fur coloration. Wings are attached to inferior extremities on the metatarsal. Calcar is well developed and keeled at 2/3 basal of its length. The tail protrudes beyond the uropatagium (ca. 5 mm).
The skull is robust, has a globular braincase with a continuous slope from the parietals to the rostrum making it look flattened in lateral view, but the posterior border is rounded. Sagittal crest present, from the middle of the sagittal suture to the interparietal, where it forms a triangular plate of bone at the intersection of the nuchal crests. Nuchal crests are well developed along the suture occipitoparietalis and between the supraoccipital and the mastoid exposure of the petrosal. Paraoccipital process well developed, wide and blunt. At the basicranium, the basisphenoid has a flattened surface, the basioccipital has a wide longitudinal ridge with two deep fosses on each side, the lateral edge of these structures forms a laminar process curved up towards the bullae reaching the posterior basicochlear commissure. Bullae are well developed. Mastoid region bulges outward from the braincase. Zygomatic arches are straight on the squamosal ramus and convergent anteriorly towards the maxillary ramus. Glenoid fossa has a rectangular shape where the width is greater than the length, with a recurved posterior edge, and anterior edge well developed. Postorbital processes of the zygomatic arches are triangular, and their base breadth is greater than their height. In dorsal view, the anterior region of the braincase has straight parietals highly convergent towards the postorbital constriction, forming an abrupt angle with the frontal borders in a conspicuous waist shape. The rostrum is shorter than the braincase (1/3 of the total length of the skull). The supraorbital region is swollen without marked postorbital ridges. The external nasal fossa has a "U" shape with a conspicuous emargination of the nasal bones. Premaxillae are robust and continuous regarding the braincase and rostrum profile. Palatine processes of the maxillae of the hard palate are visible from the dorsal view. In the ventral view, the hard palate is deeply convex, and the posterior edge of the hard palatal has a spine forming an "M" shape. The hamular processes are thickened but straight. The dental formula is I 2/3 C 1/1 P 1/2 M 3/3, with a total of 32 teeth. The upper tooth row is straight. The inner upper incisors have convergent tips, each tooth has two asymmetrical cuspids. The position of I2 is beside or lateral to I1. The height of I2 reaches 2/3 of I1 height. Each upper incisor has a conspicuous cingulum around the tooth crown. Upper canine well developed, simple without accessorial cusps, but with a cingulum rounded the crown base. The upper premolar has a continuous cingulum around the crown base, without an anterior projection. Upper molars dilambdodont. The M1 has a well-developed protocone but blunt, the paracone is shorter than the metacone, reaching 2/3 of the size of the metacone. No stylar cusps are present. The M2 has a similar pattern and size as M1. The M3 has an "N" form without metastyle. Mandibular ramus is straight with an upward inflection below the coronoid process. The angular process has a laminar form, it is longer than the condylar process, projected outward, outside of the condylar process plane. The coronoid process is taller than wide with a rounded tip curved forward. The anterior edge of the process is straight and forms a 90° angle with the dentary. Lower incisors are trilobed and crowded one after the other.
Canine is curved inward; it has an evident cingulum around the crown that is obscured by the incisors and the anterior premolar cingulum. The lower canine has an accessory cusp on the postero-lingual extreme of the cingulum, near to the contact zone with the anterior lower premolar. The anterior lower premolar is shorter than the posterior lower premolar. The posterior lower premolar has a well-developed cingulum around the crown that forms a small accessorial cusp on the antero-lingual region, and a convex emargination on the labial side near the middle of the tooth. Molars height is similar to that of the posterior premolar, but the cusps of the last molar are narrower. Anterior lower molar overlaps with the posterior cingulum of the posterior lower premolar.
Comparisons. Histiotus cadenai can be differentiated from H. velatus and H. diaphanopterus in having a rounded ear tip (fine and acute in the last two species). Additionally, H. cadenai is distinct from H. diaphanopterus in having black ears and wing membrane (vs. white translucid ears and wings in H. diaphanopterus). Cranially, H. cadenai has a highly robust cranium and swollen rostrum, and short hard palate being subequal to the size of M2, whereas in H. velatus and H. diaphanopterus the skull is narrower in dorsal view because the supraorbital region is poorly developed, and the hard palate is long projecting more than two times the size of M2. H. cadenai, H. magellanicus, H. colombiae, and H. montanus lack a connective band between ears that is characteristics of H. velatus, H. di-aphanopterus, H. alienus, H. macrotus, and H. laephotis. Histiotus cadenai is differentiated from H. magellanicus by its light brown color (dark brown dorsal and ventral coloration of H. magellanicus). In addition, H. cadenai has a developed sagittal crest only between the posterior side of interparietal bones and the lateral view of the cranium forms a continuous slope, whereas in H. magellanicus the cranium has a highly developed sagittal crest extending from the postorbital constriction to the posterior edge of the interparietal bones, and in lateral view, the rostrum and the braincase form an angle. Histiotus cadenai is differentiated from H. montanus by triangular pinnae with rounded tips compared with the ovate pinnae with pointed tips with the two sides unequal of H. montanus. Cranially, in H. cadenai the braincase and the rostrum form a continuous slope; the supraorbital region is swollen without marked postorbital ridges; the paraoccipital process is well developed, wide and blunt; the position of I2 is lateral to I1; the angular process has a laminar form and projected outward, outside of the condylar process plane; whereas in H. montanus the braincase and the rostrum form an obtuse (around 160-170°) angle; the supraorbital region is swollen with marked postorbital ridges; paraoccipital process is very well developed but projecting further back from in dorsal view; the position of I2 is behind to I1; the angular process has a laminar form, and, projected upward in the same plane of the condylar process.
Histiotus cadenai only co-occurs with H. colombiae and H. humboldti. All three species can be externally differentiated by the shape of the pinnae which is triangular with rounded tips and a notch on the outer edge in H. cade-nai, triangular with rounded tips and smooth outer edge near to the tip in H. humboldti, and ovate with asymmetric tips (two sides unequal), and the posterior edge of the tip of the pinna with a notch in H. colombiae. Additionally, H. cadenai can be differentiated from H. humboldti by the tragus shape that is ensiform (sword shape with parallel edges and acute tip) in H. cadenai, whereas the tragus of H. humboldti is eccentric with the principal axis in one side at the middle point of the structure. Furthermore, H. cadenai has a keeled calcar without lappet (lappet present in H. humboldti).
Cranially, H. cadenai is easily distinguished from H. hum-boldti because the braincase and the rostrum of H. cadenai form a continuous slope in lateral view, whereas in H. hum-boldti the braincase and the rostrum form an angle (Figs. 4 and 5). H. cadenai has a developed sagittal crest that forms a triangular plate of bone at the intersection with the nuchal crests (not developed sagittal crest and the braincase is rounded without a bone plate in H. humboldti). The para-occipital processes are well developed in H. cadenai, but wide and blunt (tubular in form and recurved downward) in H. humboldti (Figs. 4 and 5). Furthermore, H. cadenai can be distinguished from H. colombiae by the upper premolar without an anterior projection in the former and the upper premolar with a well-developed anterior projection in the latter. The supraorbital region of H. cadenai is swollen without marked postorbital ridges whereas H. colom-biae has marked postorbital ridges. In H. cadenai the I2 is beside or lateral to I1, whereas in H. humboldti and H. colombiae the I2 is behind the I1. Finally, the angular process in H. cadenai has a laminar form and is projected outside the condylar process plane, whereas in H. humboldti the angular process has a tubular form and is projected upward in the same plane of the condylar process, and in H. colombiae has a laminar form, projected upward and outside, but in the same plane of the condylar process (Figs. 4 and 5).

Figure 4 Dorsal and ventral views of skull and lateral view of skull and mandible of the holotype of Histiotus cadenai sp. n. (ICN 16980). Scale bar = 10 mm.

Figure 5 Dorsal and ventral views of skull and lateral views of skull and mandible of a, e. H. humboldti (ICN 16979). b, f. H. cadenai sp. n. (ICN 16980). c, g. H. colombiae (IAvH 8598). d, h. H. montanus (MHNG 93314). Scale bar 10 mm. 1. The braincase and the rostrum form a continuous slope. 2. Sagittal crest. 3. Supraorbital region. 4. Paraoccipital process. 5. I2 is beside or lateral to I1. 6. Upper premolar with a well-developed anterior projection. ICN: Instituto de Ciencias Naturales; IAvH: Instituto Alexander von Humboldt; MHNG: Muséum d'Histoire Naturelle de la Ville de Genève.
The specific recognition of Histiotus colombiae
Our analyses clearly showed the validity of at species level of H. colombiae based on acoustic, morphological, and genetic characters and clarified that the nominal H. montanus is distributed in Argentina, Chile and Uruguay. For these reasons, we present the information on the systematics of this taxon:
Family Vespertilionidae Gray, 1821
Genus Histiotus I. Geoffroy Saint-Hilaire, 1824
Histiotus colombiaeThomas, 1916
Histiotus montanus colombiae:Cabrera, 1958:109; name combination.
Histiotus montanus colombiae:Handley and Gardner, 2008: part.
Histiotus montanus:Rodríguez-Posada 2010: 176, not Vespertilio montanus Philippi and Landbeck, 1861.
Type locality: "Choachi, near Bogota," Cundinamarca, Colombia.
Holotype: British Museum of Natural History (BMNH 99.11.4.1.) ♀ preserved as skin and cleaned skull (skull broken), collected on 20 August 1895.
Description. H. colombiae has a dorsal light brown color with the tips lighter than the base. The ventral coloration also presents two phases and is much lighter than the dorsal. The band connecting the ears is not evident; the shape of the pinnae is triangular ovate with asymmetric tips (two sides unequal), and the posterior edge of the tip of the pinna with a notch. The skull is robust (GLS: 17.67-18.72 mm) and has a wider interorbital zone than other species such as H. laephotis and H. alienus. The upper premolar of H. colombiae has a well-developed anterior projection (see Thomas 1916). The supraorbital region of H. colombiae has marked postorbital ridges. The I2 is behind the I1. The angular process has a laminar form, projected upward and outside, but in the same plane of the condylar process (see Table 2 in the supplementary material for morphological comparison with additional Histiotus species).
Distribution of Histiotus in Colombia
Histiotus cadenai is distributed from the Cordillera Central in Colombia to the north of Ecuador, over 2550 m (Fig. 6a). The distribution of H. colombiae encompasses the high plains of the Cordillera Oriental in the departments of Boyacá and Cundinamarca in an elevational range between 2600 and 3100 m (Fig. 6a). Finally, H. humboldti has the widest distribution covering the three Cordilleras of the Colombian Andes and the north of Ecuador in an eleva-tional range between 1700 and 3000 m (Fig. 6b).
DISCUSSION
The new species and the recognition of H. colombiae increase the number of recognized species of Histiotus from eight (Moratelli et al. 2019) to ten, with three of them occurring in Colombia. Despite that, we suggest that number of species in Histiotus is larger because the samples from Peru and Bolivia remain unrevised. In ours and in previous assessments of the diversity of Histiotus (Gimenez et al. 2019) one unidentified specimen from Peru (AMNH 278521) is included as a potential undescribed species. In addition, our review of the holotype of H. m. inambarus from Peru showed a particular cranial morphology and might represent a distinct valid species. The validation of these hypotheses should be subject of further studies with the inclusion and comparison of new material.
The inclusion of a taxonomic framework based on different sources of evidence is needed to clarify the diversity of the genus and will facilitate species delimitation. Unfortunately, taxonomic assessments are challenging because acoustic or genetic data are not available for most Histiotus species, and there are few specimens in natural history collections. For instance, the northern South American forms had not been studied since Thomas (1916) and Handley (1996) descriptions, perhaps due to the scarcity of specimens. Although Rodríguez-Posada (2010) called attention to the potential novelties on the diversity of Colombian Histiotus, it took a decade to complete morphological, genetic, and acoustic data to compare northern specimens with the southern form (Ossa et al. 2015, Gimenez et al. 2019). The easiest way to solve this situation is the collaborative work, joining resources and capacities of Neotropical mammalogists that work along with the countries where these species are distributed.
Finally, our findings increase the number of bat species of Colombia to 217 species (23 vespertilionids), including recent records for the country (Basantes et al. 2020, Morales-Martínez et al. 2020), and a new species, Vampyressa voragine, described recently (Morales-Martínez et al. 2021). We highlight the urgent need for systematic reviews of poorly sampled groups of bats, considering that they have shown an increase in the number of validated taxa based on morphological (Moratelli et al. 2013, Tavares et al. 2014, Mantilla-Meluk and Montenegro 2016), and molecular data (Loureiro et al. 2018). This is particularly important in areas like the northern Andes that are considered a highly threatened biodiversity hotspot (Myers et al. 2000).





















