Introduction
The Carajás National Forest is a mountainous complex located in the State of Pará, Brazil, in the southeastern plain of the Amazon (Zanetti et al., 2020; Silveira et al., 2016; Babiychuk et al., 2017). There, a unique ecosystem known as Canga is characterized by rugged relief, the presence of plateaus of ferruginous rocks, and abundant mineral resources (Viana et al., 2016; Devecchi et al., 2020). The Canga has significant economic relevance because of its mining potential and peculiar ecological environment, which includes endemic species threatened by extinction (Schaefer et al., 2015; Viana et al., 2016).
Various species of the Ipomoea genus in the Canga habitat have already been recorded (Viana et al., 2016; Mota et al., 2018). Among the endemic plants in this area, Ipomoea cavalcantei, popularly known as the Carajás flower, is noteworthy (Zappi, 2017). This species is a liana belonging to the Convolvulaceae, which has approximately 1900 species divided in 59 genera that are geographically distributed in all tropical regions (Simoes et al., 2017). Several species of Convolvulaceae in tropical regions are of ornamental, medical, and nutritional importance (Nkumah et al., 2015), while others are toxic or invasive plants (Bezerra et al., 2022).
Currently, the natural populations of the Carajás flower are threatened because their habitats are often place of mining activities (Viana et al., 2016). Statistical analyses have already shown the impact of mining on the habitat of I. cavalcantei, considering a 50 % loss of this territory in the last three decades (Rodrigues et al., 2020). This substantial decline not only impacts the reduction of the species’ spatial distribution, which is already restricted, but also leads to genetic variability losses, as the entire population is suppressed by economic activities (Martinelli & Moraes, 2013; Simáo-Bianchini et al., 2016). Some studies have already evaluated the natural history of the species and its relationship with other Ipomoea species that occur in the area (Babiychuk et al., 2017). However, given the great risk of extinction and the absence of a scientific history of the reproduction of the species under ex situ conditions, it is necessary to develop long-term conservation strategies for I. cavalcantei, as in situ conservation alone may be insufficient (Joppa et al., 2013).
Researches that contribute to the consolidation of knowledge regarding fruits and seeds are necessary to provide important information for ex situ reproduction strategies for certain species, especially, endangered endemic species (Miller et al., 2017; Zanetti et al., 2020). Biometric studies indicate the genetic variability within natural populations and the relationship between this variability and abiotic factors, which are useful for the conservation and exploitation of plant resources (Gongalves et al., 2013). Determining the biometric characteristics of seeds can improve the management of protected areas or their sustainable use, as they provide information for future studies regarding characteristics of the species and their proper identification (Souza et al., 2019).
The Carajás flower is located in an environment with specific climatic characteristics, and the physiological quality of the seeds and their germination characteristics are essential information for conservation programs and germplasm banks, thus, new information regarding the species is essential for establishing protocols and routines (Clemente et al., 2017). Therefore, the aim of this study was to verify the impact of different methods of overcoming dormancy on the germination of I. cavalcantei seeds, and to survey the biometric data of its seeds and fruits. It is expected that the results will contribute to ex situ I. cavalcantei conservation strategies.
Material and methods
Fruít and seed sample
The fruits were collected from populations of I. cavalcantei in the Carajás National Forest (FLONACA), Southeast Pará State (58°06’73.0” S and 93°32’07.1” W). The fruit collection took place at random in June 2019, covering the areas N1 and N2 in the Canga habitat (Figure 1). This collection was authorized by the Instituto Chico Mendes de Conservado da Biodiversidade (SISBIO 67760-1). According to the Koopen Climate Classification, the climate in this region is “AWi” type (Hartwig, 2016), indicating a tropical rainy climate with winter drought, in which the annual rainfall varies between 2000 mm and 2400 mm, and the monthly average temperature is greater than 18 °C. During the fruit processing, heavily damaged seeds (predated by insects or poorly developed) were removed. Before analysis, the seeds were disinfected with fungicide Captan® 5 % for five minutes.
Fruít and seed bíometrícs and moísture degree.
The biometric characterization was performed after processing the fruits and seeds, with a sampling of 100 fruits and 100 seeds. The variables measured were: i) length, width, and thickness (mm), measured using a digital caliper with a degree of accuracy of ± 0.01 mm; and ii) weight (g), measured using a digital analytical balance with a precision of 0.001 g. With the obtained data, it was possible to estimate the minimum, maximum, average, standard deviation, and coefficient of variation (CV) of the samples. The moisture content was determined according to the methodology described by Brasil (2009), using three samples of 10 seeds that were weighed and dried. The results obtained were expressed as percentages.
Germínatíon tests
The six pre-germination treatments included mechanical, physical, and chemical methods, as summarized in Table 1. After the pre-germinative treatments, the seeds were placed in petri dishes on two sheets of paper towels (Germitest®) moistened with distilled water, at a proportion of 2.5 times the weight of the dry paper (Brasil, 2009). The treatments were placed in a biochemical oxygen demand chamber, regulated at a constant temperature of 25° C and a photoperiod of 12/12 h (light/dark). All treatments were designed in completely randomized blocks and replicated five times with 20 seeds each, totalizing 100 seeds per treatment.
Assessments were performed daily until day 18. The results were expressed as the total germination percentage (G %) on day 10, the germination speed index (GSI), and the average germination time (AGT). Seeds that emitted radicles equal to or greater than 2 mm were considered germinated (Brasil, 2009). The GSI was calculated as the sum of the number of seeds germinated each day, divided by the number of days elapsed between sowing and germination (Maguire, 1962), and the AGT was expressed in days, obtained from the daily counts of the germinated seeds immediately after sowing until the moment of analysis (Labouriau, 1983).
Water absorption
To conduct the water absorption test for the seeds, the following treatments were performed using five replicates of 10 seeds for each treatment: i) control (E1), wherein no methods were applied to the seeds; and ii) mechanical treatment with sandpaper scarification (E2). The seeds were previously treated with a proportion of 5 % fungicide Captan® in a beaker containing 500 mL of water, and then weighed. After treatment, they were kept under the same conditions as those used for the germination test. During the analysis period, the seeds were removed from the dishes and superficially dried using a paper towel. The seeds were weighed every two hours during the first 24 h, after which measurements were conducted every 12 h until there was root emission in at least 50 % of the seeds (on day 9).
Statistical analysis
The resultant percentage values were transformed using the square root arcsine of the germination percentage to normalize the data before applying the statistical tests. The data was evaluated through an analysis of variance, which was used to test significant sources of variation, and later the least significance difference test was applied to compare means. The probability level used for statistical significance was 5 %. Statistical and graphical analyses were performed using a R-3.5.1 software.
Results and discussion
Seed and fruit biometric data
For the I. cavalcantei seeds, the moisture content was 13.03 % immediately after field collection and manual processing, and the weight of the 100 seeds was 2.80 g. For the seeds, the CV results were 10.76 %, 10.95 %, and 24.09 % for the length, width, and thickness, respectively. The general descriptive statistics for the fruit and seed variables are listed in Table 2.
Table 2 Descriptive statistics for the biometric parameters of Ipomoea cavalcantei fruits and seeds
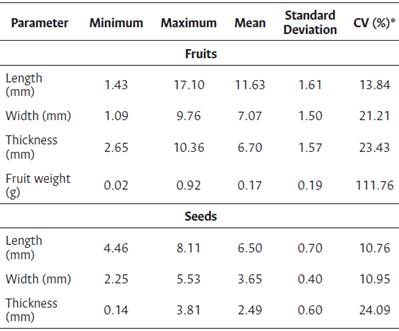
*CV = coefficient of variation.
This species has no ex situ replication, and it is only possible to collect biological material in its natural habitat (in situ), where fruits and seeds are subject to many factors (biological, climatic, environmental), which are sources for the variation of biometric data. So, the variation in the seed length, width, and thickness was similar to that observed for fruit. However, the CV of the fruits weight showed greater variability (CV of 111.76 %). Biometric data is important to identify genetic variability within populations of the same species, and can provide relevant data to distinguish species of the same genus (Gusmao et al., 2006), as well as to obtain information on the differentiation of species of the same genus that grow in the same environment (Santos et al., 2019)width, thickness, mass of fruit and seeds were measured. Seeds underwent the following pre- germination treatments: seeds without any treatment (control. The differences in the biometric results of fruits and seeds obtained in this study may be associated with genetic and environmental factors, which influence the period between flowering and fruit ripening (Souza et al., 2016).
In a study on the typical arboreal species of the Cerrado Magonia pubescens,Macedo et al. (2009) observed that the biometric variations of fruits and seeds may be influenced by environmental factors during flowering and plant growth, which may indicate high variability of population genetics. Considering the existence of more individuals of the Ipomoea genus in the Canga-like habitat (Simáo-Bianchini et al., 2016), the biometric data will contribute to the identification and comparison of different species, and will provide a basis for comparing in situ and ex situ characteristics.
Germination data
In this study, pre-germination treatments on seeds of I. cavalcantei were performed to identify the best method to overcome seed dormancy (Figure 2). Treatments T3 and T6 started to germinate on the first day, and the maximum rate of increase in germination occurred between days three and four.

Figure 2 Germination percentage of I. cavalcantei submitted to different treatments to overcome dormancy. T1: Control (no treatments); T2: Mechanical scarification with sandpaper; T3: Mechanical scarification with sandpaper and immersion in water at 25° C for 12 min.; T4: Mechanical scarification with sandpaper and water bath at a temperature of 60 °C for 5 min; T5: Immersion in sulfuric acid (H2SO4) for 1 min; T6: Immersion in water at temperature of 60 °C for 60 min.
Some species of the genus Ipomoea may have dormancy because of the impermeability of the tegument to water (Azania et al., 2009). Therefore, the efficiency of the treatments in this study is related to exposing the seed tegument to friction with sandpaper, which helped the seed absorb water. This absorption of water contributes to tissue hydration and increases respiration and metabolic activities, which results in higher demands for energy and nutrients, consequently stimulating seed germination (Carvalho & Nakagawa, 2000; Wagner & Oplinger, 2017).
Santos and Salomáo (2019) verified the efficiency of the scarification method to overcome dormancy in Ipomoea cynanchifolia seeds, which resulted in increased germination rates. Azania et al. (2003) achieved better results using a mechanical scarification method, with an increase in the percentage of germination for I. grandifolia, I. hederifolia, I. quamoclit, I. nil, Merremia cissoides, and M. aegyptia.Pazuch et al. (2015) also found that mechanical scarification favored better germination rates for I. grandifolia, I. purpurea, and I. indivisa seeds.
Treatments T1 and T6 had the longest average time to germinate. Specifically, the T6 treatment exhibited an intermediate percentage of dormancy in the I. cavalcantei seeds. The results of Pazuch et al. (2015) demonstrated that treatment with hot water did not satisfactorily influence the germination process of the I. purpurea seeds. Our data showed that scarification is the recommended pre-germinative treatment method for I. cavalcantei, as compared with other treatments (Table 3).
Table 3 Average results of germination percentage at 10 days (G%), average germination time (AGT), and germination speed index (GSI) of
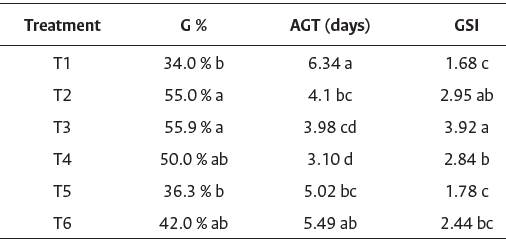
T1: Control (no treatments); T2: Mechanical scarification with sandpaper; T3: Mechanical scarification with sandpaper and immersion in water at 25° C for 12 min.; T4: Mechanical scarification with sandpaper and water bath at a temperature of 60 °C for 5 min; T5: Immersion in sulfuric acid (H2SO4) for 1 min; T6: Immersion in water at temperature of 60 °C for 60 min. The same letters in the same column indicate no statistical difference between treatments. Least significance difference (LSD) test of means (p < 0.05).
Treatment T1 presented the lowest GSI in relation to the other treatments and, consequently, had a longer AGT. The means of the GSI values obtained in treatments T1, T5, and T6 did not show a significant difference, with a smaller number of seeds germinated daily. Mechanical and chemical scarification and immersion in hot water generate small openings in the tegument, thereby increasing permeability and allowing imbibition (Maldonado- Arciniegas et al., 2018).
Regarding treatment T5, we did not observe large increases in germination after nine days. In addition, the low germination percentages acquired with chemical scarification may be caused by the exposure time to sulfuric acid that was not sufficient to break the tegument, delaying water absorption, and consequently the protrusion of the radicle. Similar results were obtained by Pazuch et al. (2015), who verified that the immersion of seeds in sulfuric acid was not efficient in increasing the germination percentage of I. grandifolia seeds. Conversely, the results obtained by Azania et al. (2003) showed that the exposure of Ipomoea seeds to sulfuric acid increased the germination percentage.
We found that T4 had a shorter AGT (3.10 days) and did not differ statistically from T3 (3.98 days). Treatments T2, T3, and T4 showed higher GSI values, exhibiting greater potential for daily seed germination. Similar results were obtained by Santos and Salomao (2019), who performed scarification with sandpaper, and obtained higher germination percentages for the seeds of I. cynanchifolia.
It is recommended to apply mainly treatments T2, T3, and T4 to overcome I. cavalcantei seeds dormancy, in order to disrupt the tegument without damaging the seeds. These treatments are recommended to accelerate the germination process, which may contribute to the efficiency in ex situ reproduction strategies.
The results of seed dormancy can also be correlated with the characteristics of the Canga environment. Immediately after the fructification period, the habitat experiences a large period of drought, and the species with deciduous characteristics only produce new branches and leaves after the return of the rainy period. In this context, physical scarification methods contribute to the efficiency and uniformity of the ex situ germination of the species, as in their habitat, the seeds can become vulnerable to attacks from predators and to the temporal limitation of fructification.
Imbibition curve
The imbibition process of the I. cavalcantei seeds resulted in a three-phase imbibition pattern (Figure 3). In the first phase (phase I), E1 and E2 showed increasing average mass values as a result of high water absorbency. The seeds scarified with sandpaper had an average initial weight of 200 mg and an average final weight of 452 mg, which is 12.4 % higher than that of the E1 treatment. The increase in water absorption in the treatment with scarification was higher in phase I, and it stabilized in both treatments after 72 hours. Imbibition results reinforce the idea that scarification is more interesting to increase the efficiency of germination.
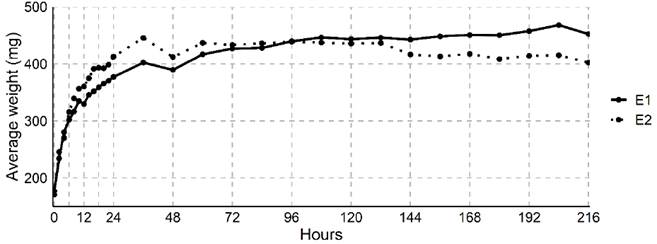
Figure 3 Ipomoea cavalcantei seed imbibition curve, expressing the average seed weight (mg) as a function of time (h). E1: No methods were applied to the seeds; E2: mechanical treatment with sandpaper scarification.
During the interval between 48 and 72 h of imbibition, there was a reduced mass gain, which can be described as a stationary stage (phase II) or physiological rest (Soares et al., 2019). After 82 h, phase III began, which is characterized by seeds with an average low weight gain. In contrast, in the scarified seeds, there was a reduction in the average weight after 127 h. Thus, phase III begins with protrusion of the radicle, which occurs only in living and non-dormant seeds.
Conclusions
The fruits and seeds of I. cavalcantei presented biometric variations in length, width, and thickness. The seeds of I. cavalcantei did not show higher resistance to water penetration in different treatments. However, scarification methods with sandpaper are recommended to increase the uniformity of time and germination percentages, thereby increasing the efficiency of obtaining seedlings of this species. Such information can be used for developing ex situ conservation strategies for species potentially threatened by advances in mining activities.
















