Colombian Journal of Anestesiology
ISSN 0120-3347
Editorial
The fiscal cliff and opportunities for change☆
El abismo fiscal y las oportunidades de cambio
Catalina Ceballos-Stendall (FRCA)a,b,*, David Walker (FRCP, FRCA, FFlCM)b,c
a Anaesthetic Registrar, Kings College Hospital, Denmark Hill, London SE5 9RS, United Kingdom
b Consultant in Anaesthesia & Critical Care Medicine
c Director, Perioperative Masters Programme, UCL Division of Surgery & Interventional Science, 4th Floor, UCL Medical School Building, 21 University Street, London WC1E 6AU, United Kingdom
☆ Please cite this article as: Ceballos-Stendall C, Walker D. El abismo fiscal y las oportunidades de cambio. Rev Colomb Anestesiol. 2017;45:1-4.
* Corresponding author at: Kings College Hospital, Denmark Hill, London SE5 9RS, United Kingdom.
E-mail address: catalina.stendall@gmail.com (C. Ceballos-Stendall).
Modern Colombia with an estimated population of 48 million inhabitants has embarked on a transition towards universal health care for its citizens. In doing so the country will inevitably face both epidemiological and financial pressures. These challenges for health policy makers in Colombia are common to all modern economies and ultimately will shape the health care service the nation delivers. Bound within this context, the quest for improved surgical outcomes for an ageing, increasingly comorbid population will require new thinking and as such represents an exciting opportunity for anaesthesiologists to engage in innovations that will improve patient care.
Inevitably as part of this process, traditional roles and responsibilities of anesthesiologists and other healthcare workers will be questioned, challenged and most likely evolved. Some have chosen to view this proposition as an existential crisis within our own profession.1 A more positive view might be that as a profession, we are displaying an appetite for change. In so doing anesthesiologists working within a dynamic eco-system, have recognised the need to adapt and evolve to ensure the longevity of our professional species.
In Colombia the current average life expectancy is 79 years and in line with most other countries, it is expected to continue to rise. In the United States, the number of patients over the age of 85 years is expected to double by 2036 and triple by 2049 and similar trends are evident throughout the developed world.2 As a consequence, the demand on age associated surgeries, e.g. oncological, orthopaedic degenerative and emergency surgery will increase. In parallel, surgical programmes of activity continue to evolve to offer new interventions where historically for many patients there had been little hope of surgical cure. This paradigm appears to be fuelling momentum for change internationally and is being addressed predominantly by anesthesiologists, who are well positioned to understand this 'surgical epidemic' and effect change.
Physiological performance declines with age while organ specific morbidity increases. As a consequence a growing population of surgical patients termed high-risk are least able to show resilience to the pathophysiological challenges of ill-health and surgical stress.3 As this cohort of the elderly, often frail and infirm patients continues to grow, they account for a disproportionate number of deaths and major morbidity in the perioperative period.4 These facts have been well understood for more that a quarter of a century, yet we have been slow to evolve from legacy systems, which have failed to adequately characterise patient risk; have adopted a 'one-size fits all' approach to care and have been broadly reactive to perioperative complications rather than proactive to their prevention.5
Colombia spends 5.41% of its gross domestic product (GDP) on health care (2014), a figure similar in percentage terms to that of the UK and many other European countries. In the United Kingdom patients have enjoyed universal health care (National Health Service (NHS)) for more than half a century, but in recent times the escalating costs of running this nationalised health industry has seen the emergence of a funding gap between actual spending and that spending required to meet the growing healthcare demand. Currently this gap is measured in billions of U.K. pounds and is estimated to continue to rise significantly during the next decade. Therefore, cost savings are appealing to both governments, insurers and tax payers alike; few could argue with the aim of delivering more for less, by means of cutting waste and leaning process as an important first step in cost reduction. In the U.K. improved efficiency has demonstrated cost savings of between 0.6 and 1.6% per annum. However this figure falls short of an estimated 4% efficiency saving per year required to balance the books. The picture becomes even more sobering when the efficiency savings are adjusted for quality metrics, e.g. survival, hospital appointment and waiting times, which have demonstrated no improvement for several years.6
Faced with health care funding deficits governments are most often challenged to improve productivity and efficiency and good examples of surgical models already exist. For more that 20 years the work of Kehlet and colleagues,7 has made popular a highly protocolised pathway of surgical care. At its core is the aim to limit the systemic inflammatory response associated with the surgical insult, rationalise interventions and limit redundancy by streamlining the patient's surgical journey. Today, such Enhanced Recovery After Surgery (ERAS) pathways are a watch-word for efficiency and represent standard of care for many patients undergoing major surgery. Most recently North American institutions have begun to adopt and adapt ERAS models made popular in Europe8 and it is likely such activity will attract much attention from healthcare insurers, in an attempt to reduce their per capita costs. Exemplar institutions, seen, as early adopters of these 'new ways' must be scrutinised, learned from and hailed as championing new opportunities in healthcare delivery. The evolution of ERAS and it's acknowledged success is in part due to the willingness of large-scale organisations to benchmark their own data, share best practice and engage in meaningful discussion for improvement.9
Whether defining clinical best practice, costing individual elements of care, managing risk or framing national health policy, a rational discussion for improvement must be informed by intelligent use of data. High quality, risk adjusted large-scale data is needed for improvement. But an understanding of what to do with the data is fundamental. The danger of poor data acquisition for research purposes can result in incomplete and difficult to analyse data sets drawing conclusions, which may not be universally translatable. At face value this is understood, but many practical impediments exist to limit such data collection and utilisation. In spite of the promise of large scale IT solutions linking patient electronic records locally, between organisations, networks and even countries, the evolution to big data analysis has been slow to evolve. Where patient data may have commercial value, further complexities exist in sharing and utilising patient episode statistics. However the ability to measure must be seen as an important first step to control, influence and ultimately improve patient care. Without a willingness to resolve the fragmentation of IT systems it is likely the inherent problems of surgery will not be identified, let alone solved.
The so-called fiscal cliff fast approaching is an opportunity to better understand what it means to commission value in surgical health care. In this environment improved surgical health must be about doing things better and even differently and less about simply doing more. The concept of less surgery may indeed challenge the conventional wisdom about what is understood by medical progress. However such a review may offer a timely opportunity to understand which elements of care are deficient, what might be improved and indeed what care might add no value for patients and health funders alike.
Seeing the surgical episode as a complete 'patient journey' must result in the breakdown of the 'silo-culture', where traditionally fragmented and often uncoordinated care has been allowed to evolve in the hospital environment. An inevitable move towards a 'bundled care payment', where a price is fixed for the whole episode of care, from admission to discharge, may be that opportunity for change. It will take us back to facts which need addressing as much today as they did a quarter of a century ago. We know why patients undergoing surgery die or suffer major complications. If we have already characterised this cohort, understand the nature of their pathophysiological decline, why then have we been slow to implement better processes and ultimately a better culture? The answer is probably we have done, but not as well as we should have.
There is an opportunity in the perioperative period for new clinical leadership, it has come about because there is an appetite for change, driven by a better understanding of the problems and encouraged by the stark realities of twenty first century health economics. In defining new ways we must not get distracted by discussions of 'patient ownership' or the territory in which we conduct our professional practice, but rather be sure we are tooled with the attitudes and skills to make a difference as a perioperative team.
No longer seen as a series of isolated interventions, the surgical episode must now be understood as a continuum of care, where the complexity of modern medicine is met with a coordinated approach to delivery. This process must be overseen by a group who have responsibility for the journey and not just the destination. Patients at the heart of our interventions must have their say in what care adds value to their life and patient reported outcomes and experiences are metrics as important as the surgical mortality. The focus on task driven health, where each contributes expertise, commanding a fee for service is seen as potentially appealing, in that it drives productivity by incentivising organisations to deliver more, but may ultimately results in a care fragmentation.
What are the likely skills needed, who will lead and what route map will be followed, are questions being addressed on each continent as subtle differences exist between health systems even though the core principles are common to all.
In the U.K., the Royal College of Anaesthetist has launched its vision for the future of perioperative care and already fuelled a national debate10 engaging all national stakeholders. Not surprisingly, the College believes that anesthesiologists will play a leading role in the pathway re-design and collaborative work with many other professional groups. It is further recognised that in rising to this challenge our profession must acquire the skills and attributes, which will define us as Perioperative Physicians. Successive generations of anesthesiologists have learned their craft, been quick to embrace technology and innovation as part of our professional identity, resulting in improved safety and outcomes for patients within the operating room. If we elect to step up to the challenges of modern surgery, the next generation of anesthesiologists will need to build on our notable past successes. Defining a syllabus where patient outcomes are explored through understanding risk and where the concept of 'failure to rescue' might be considered a consequence of poor sytem design and staff engagement will be central components of new learning.
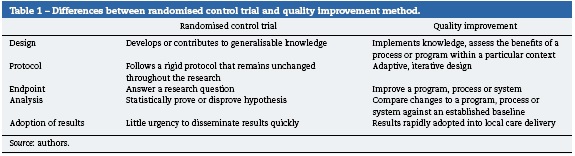
We all have a part to play and benchmarking our progress by small-scale local data collection by those health workers on the ground will be an effective method for service improvement. The Royal College of Anaesthetist have used so-called quality improvement (QI) methods to measure deficiencies and ultimately effect change in practice and encourages all its members to actively engage in QI. In the U.K, as part of a doctor's annual appraisal for fitness to practice, they must each demonstrate engagement with QI activity. QI projects are designed to implement knowledge, improve programs and assess the benefits of a process within a particular context. The data collected will help to modify already established processes and can rapidly be adopted to modify care delivery. Their methodology differs significantly from the randomised control trial, in that it takes acknowledged best practice and assesses local compliance and where deficient a documented process of iterative change is instituted11 (Table 1).
The future for surgical programmes operating in fiscally constrained economies and facing a growing burden of disease must look to new ways to deliver safe and effective care. There must be a reliance on data driven improvement, whether at national or local level and an energy to consider different methods of care delivery. In so doing we must hold on to what is good, challenge what is not and put patients at the centre of what we do. As a Nobel Laureate once said, 'the times they are a changin'.
Funding
The authors did not receive sponsorship to undertake this article.
Conflicts of interest
David Walker is a Director of a medical education programme in Perioperative Medicine.
References
1. Census.org [Internet]. United Stated Census Bureau [cited 2016 May 14]. Available from: https://www.census.gov/population/projections/data/national/2012.html. [ Links ]
2. McGlennan A, Dinner L, Drewery H, Shaw C. Anaesthesia's existential crisis. RCoA Bulletin. 2015;93:20-2. [ Links ]
3. Turrentine FE, Wang H, Simpson VB, Jones RS. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg. 2006;203:865-77. [ Links ]
4. Campling EA, Devlin HB, Hoile RW, Lunn JN. The report of the National Confidential Enquiry into perioperative deaths 1990. London: National Confidential Enquiry into Perioperative Deaths; 1992. [ Links ]
5. EuSOS [Internet]. European Surgical Outcome Study [cited 2016 Jun 26]. Available from: http://eusos.esicm.org/ study-documents/. [ Links ]
6. NHS England [Internet]. UK NHS five year forward view. [cited 2016 July]. Available from: https://www.england.nhs.uk/ourwork/futurenhs/nhs-five-year-forward-view-web-version/5yfv-exec-sum/. [ Links ]
7. Møiniche S, Bülow S, Hesselfeldt P, Hestbaek A, Kehlet H. Convalescence and hospital stay after colonic surgery with balanced analgesia, early oral feeding, and enforced mobilisation. Eur J Surg. 1995;161:283-8. [ Links ]
8. ERAS society.org [Internet]. Eras Society. List of guidelines [cited 2016 Sep 19]. Anaesthesia documents [about 2 screens]. Available from: http://erassociety.org.loopiadns.com/guidelines/list-of-guidelines/. [ Links ]
9. Maessen JMC, Dejong CHC, Kessels AGH, von Meyenfeld MF, Enhanced Recovery After Surgery (ERAS) Group. Length of stay: an inappropriate readout of the success of enhanced recovery programs. World J Surg. 2008;32:971-5. [ Links ]
10. Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC et al. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295-304. [ Links ]
11. The children Hospital of Philadelphia Research Institute [Internet]. Quality improvement vs. Research [cited 2016 Sep 29]. Available from: https://irb.research.chop.edu/quality-improvement-vs-research. [ Links ]