Introduction
Sodium is the main ion in extracellular fluid, with normal plasma values ranging between 135 and 145 milliequivalents per liter (mEq/L). It is also the main determinant of serum osmolarity, important for fluid transport across the cell membrane and nerve impulse transmission at the time of cell depolarization.1
Hyponatremia is defined as a serum sodium concentration below 135 mEq/L and may occur, in general, when water intake or administration exceeds elimination or when sodium loss exceeds intake. This determines whether hyponatremia is classified as hypovolemic, normovolemic, or hypervolemic.2 According to plasma levels, it is classified as normal 135 to 145, mild 130 to 134, moderate 125 to 129, or severe when levels are below 125 mEq/L.3 Hospitalized patients are exposed to various factors that alter homeostasis, including the use of different medications, pain, organ failure, and inadequate fluid supply. Consequently, ion balance maintenance will depend on the integrity and capacity of the autoregulation systems. Hyponatremia is the most common electrolyte imbalance in hospitalized patients, and it is even more frequent in patients with some degree of neurologic impairment.1,2 Neurosurgical patients suffer frequently from this disorder; moreover, the presence of low serum sodium levels postoperatively has been associated with an increase in mortality.4
The prevalence of hyponatremia in patients hospitalized for general causes is as high as 20%, and in patients with neurosurgical disorders it is as high as 50%.5 In patients with subarachnoid hemorrhage, hyponatremia has been reported to occur in 30% to 40% of cases,6 and the association of the 2 conditions leads to cerebral ischemia which, in turn, gives rise to higher mortality rates and worse outcomes on the Glasgow coma scale.7 On the other hand, in patients with bacterial meningitis, rates are as high as 36% to 58%, and can reach 70% in patients with tuberculous meningitis. Different hyponatremic disorders also occur in patients with brain tumors, hydrocephalus, and subdural hematomas.8,9 Close to 15% of patients with head injury develop hyponatremia, usually within the first 5 days following trauma, extending length of stay.10 In patients with head injury, sodium disturbances may be poorly tolerated, secondary to cerebral edema and alteration of adaptive mechanisms,4 increasing short-term and long-term mortality rates.10
In patients with a neurological disorder, 2 entities playa key role as triggers of hyponatremia: cerebral salt-wasting syndrome (CSWS), and the syndrome of inappropriate anti-diuretic hormone secretion (SIADH). These syndromes are directly related with altered mechanisms of osmolarity regulation between the brain and the kidney.9
SIADH is a volume expansion state due to increased renal water re-uptake mediated by excess and inappropriate anti-diuretic hormone secretion.11 This results in increased glomerular filtration rate, leading to lower sodium reuptake in the proximal tubule, increasing its urinary excretion12; there is also a reduction in serum levels of uric acid and blood urea nitrogen (BUN) which cotransport with sodium. This creates a state ofdilutional hyponatremia in patients clinically hypervolemic or euvolemic. SIADH is associated with different neurological conditions such as encephalitis, meningitis, and head injury, as well as postoperative states following brain tumor removal and trans-sphenoidal pituitary surgery.13
CSWS is characterized by excess renal loss of sodium, accompanied by volume depletion, leading to increased diuretic activity.14 Natriuretic peptides, in particular brain natriuretic peptide, associated with a reduction in sympathetic flow secondary to neurological disorders, play a key role in the pathophysiology of this syndrome.9 Sympathetic tone acts through control over renin release in juxtaglomerular cells and, in reducing their action, sodium and water reuptake in the proximal tubules is diminished. In turn, natriuretic peptides stimulate afferent arteriole dilatation and efferent arteriole vasonconstriction, increasing glomerular filtration rate; they also act on collecting tubules, inhibiting angiotensin II-stimulated sodium transporters and counteracting the renal action of arginine vasopressin.15
Causes of hyponatremia other than CSWS and SIADH include adrenal insufficiency and hypthyroidism, which must be treated with hormone replacement accordingly. In patients with hyponatremia secondary to gastrointestinal losses, fluid resuscitation and diuresis stimulation are indicated.8
The clinical manifestations of hyponatremia are related to its severity, with symptoms usually appearing when plasma sodium levels are lower than 120mEq/L, and also with insaturation velocity, because in processes in which hyponatremia develops slowly, low sodium concentrations may be achieved without the presence of brain edema, thus reducing the frequency and severity of the symptoms. Symptoms include headache, anorexia, nausea, vomiting, and muscle weakness, leading quickly to neurological complications such as brain edema, seizures, apnea, coma, and death from brain herniation, when timely treatment is not provided.15
There are no studies at present in our institution or the region about the incidence or the behavior of these electrolyte imbalances in patients taken to the intensive care unit (ICU) following a neurosurgical intervention. This fact prompted the decision of studying the clinical behavior in a sizeable group of patients receiving anesthesia care at the Hernando Moncaleano Perdomo University Hospital (HUHMP) in the city of Neiva and acting as referral center to improve and optimize comprehensive management in the preoperative phase, during the surgical intervention, and during the postoperative period in neurosurgical patients.
Methodology
Descriptive and observational study conducted in the operating theaters and adult ICU of the HUHMP in the city of Neiva, Colombia during the time period between January 1, 2014 and December 31, 2015.
The population consisted of patients over 18 years of age taken either to elective or urgent surgery because of intracranial pathology and who also received postoperative care in the adult ICU during at least 72 hours. Data collection was done through direct non-participatory observation, prior endorsement of the institutional Ethics/Bioethics and Research Committee.
The analysis included demographic, anthropometric, clinical, and paraclinical variables, as well as the neurological outcome. Central trend and scatter were calculated for continuous variables, and relative and absolute frequencies were derived. The x2 or the exact Fisher test were used as statistical confidence tests, and the Kruskal-Wallis test was used to compare ordinal variables. In all cases, statistical significance was considered to be present when the P value was <0.05. Odds ratio (OR) was calculated to establish the association between the presence or absence of hyponatremia and clinical and perioperative variables. The data were entered into a matrix created in Microsoft Excel 2013 (Microsoft Corporation) and processed using the SPSS version 23 statistical package (International Business Machines Corporation [IBM] SPSS Statistics 23.0; Armonk, NY, USA).
Results
A total of 525 were intervened by the neurosurgery service during the study period and, of these, 79 were included in the study. Of the total number of patients, 73.4% were males. Median age was 40 years (range 18-85). Table 1 shows the demographic and anthropometric variables of the patients included in the study.
Table 1 Demographic and anthropometric variables.

* Age ranges according to the vital cycle of the Colombian Ministry of Health and Social Protection.
† BMI=body mass index expressed as the square of weight (kg) over height
(m).
Source: Authors
The most frequent diagnosis requiring the surgical intervention was head injury in 62%; 21.5% of the patients had a space-occupying lesion (right frontal glioma, meningioma, and primitive neuroectodermal tumor), 11.4% had non-traumatic subarachnoid hemorrhage, and 5.1% of patients were classified as having other diagnoses (brain abscess, non-traumatic subdural hematoma, toxoplasmosis, and Chiari malformation). In terms of the American Society of Anesthesiologists (ASA) classification, 55.7% of the patients were ASA IV, 20.3% ASA III, and 24.1% ASA V. The median value on the Glasgow coma scale at the time of the neurological assessment upon arrival at the operating room was 6 points. Balanced anesthesia was used in 98.7% of cases. Only 1 patient received total intravenous anesthesia, and in no patients was the pure inhaled technique used for anesthesia maintenance. Median surgical time was 120minutes (range 45-520minutes). Of all the patients, 89.9% were admitted to the ICU with a secured airway, whereas only 10.1% left the operating room breathing spontaneously.
In terms of perioperative fluid management, mannitol was the hyperosmolar therapy administered to 60.7% of the patients, whereas 43% of them received hypertonic saline solution, although it must be noted that a dual hyperosmolar therapy was used in some patients. On the other hand, during postoperative care in the ICU, the use of hyperosmolar solutions was more frequent than in surgery, at 79.7% for each solution.
The incidence of hyponatremia was 25.3%, with mild hyponatremia found in 60% of the cases, moderate hyponatremia in 15%, and severe hyponatremia in 25%. Patients with a diagnosis of subarachnoid hemorrhage were 8 times more likely to develop hyponatremia (P = 0.007) (OR 8.0; 95% confidence interval [CI]: 1.777-36.018). Regardingthe diagnosis of characteristic hyponatremic syndromes found in patients with neurological disorders, the incidence of CSWS was 5% and the incidence of SIADH was 10%. The mean duration of hyponatremia during the study period was 38 hours, and in terms of the specificpharmacological treatment for these syndromes, only 1 patient was given fludrocortisone, whereas no patient was treated with vasopressin receptor antagonists during the study period. Table 2 shows the perioperative variables of the patients in accordance with the presence or absence of hyponatremia.
Table 2 Perioperative variables categorized in accordanc e with the presence of absence of hyponatremia.

CI=confidence interval; OR=odds ratio; TIVA=total intravenous anesthesia.
* Fisher exact test.
† x2.
Source: Authors.
Hyponatremia-associated mortality was 35%, similar to overall mortality which was 35.4%. Final patient status was assessed based on neurological outcome variables using the extended Glasgow outcomes scale at discharge and in-hospital mortality, and no differences were found when comparing these final conditions in terms of the presence or absence of hyponatremia (Fig. 1), in terms of the severity of hyponatremia (Table 3) or in terms of the clinical impact of patients taken to surgery due to neurological pathology (Table 4).

Source: Authors.
Figure 1 Analysis of mortality and neurological outcomes in patients with and without hyponatremia.
Table 3 Analysis of mortality and neurological outcomes in accordance with the severity of the hyponatremia.
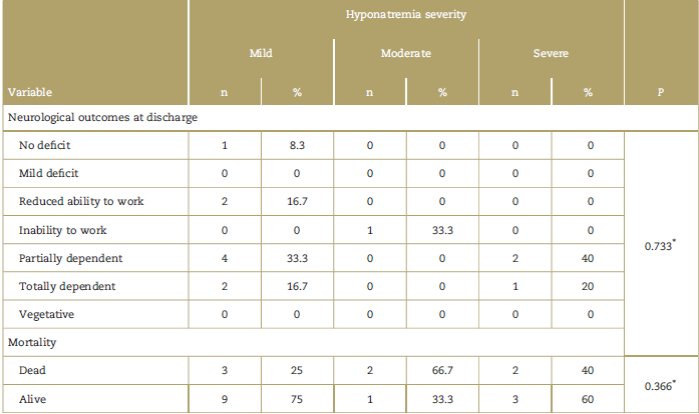
* Kruskal-Wallis.
Source: Authors.
Table 4 Clinical impact on patients taken to surgery due to a neurological pathological condition.

GOS=Glasgow Outcomes Scale.
Source: Authors.
Of the 51 patients who survived at the end of their hospital stay, 27.4% were classified as having a mild neurological deficit or no deficit. On the other hand, 11.7% of the patients showed total or partial inability to return to their work and social activities, whereas 52.9% were in a situation of dependence, either total or partial, for basic activities; 7.8% of patients were classified as being in a vegetative state, emphasizing that hyponatremia was not present in any of these cases.
The specific analysis of patients diagnosed with SIADH and CSWS did not find differences in terms of the severity of hyponatremia, mortality rates, or neurological outcomes at discharge.
Discussion
During 2014 and 2015, the neurosurgery service of HUHMP performed 525 surgical procedures. After excluding patients under 18 years of age and patients taken to procedures that did not involve intracranial pathology, only patients who received postoperative care in the ICU were selected, considering that daily measurements of electrolyte, fluid balance, and other tests needed for this study are not performed on the wards. Consequently, the study included 79 patients with intracranial neurosurgical pathology, that is, a 15% non-random sample of the total number of patients taken to surgery. Given that they are subgroup with special characteristics, the results obtained cannot be extrapolated to settings other than critically ill neurosurgical patients.
The evaluation of the characteristics of the patients included in the study found a greater proportion of male patients (73.4%) and a mean age of 40 years. This relates to the fact that head trauma was the most common reason for surgery (62%), given that male patients under 45 years of age are more prone to these injuries.16 The University Hospital where this study was conducted is the trauma referral center in the city of Neiva, which explains the higher frequency of patients with this diagnosis at the time of admission. Due to the selection criteria, no patient was classified as ASA I or II upon admission to the operating room, considering in all cases that even if many young patients had no comorbidities, the neurological injury implied some degree of functional limitation, more than half were classified as having a life-threatening condition (55.7% ASA IV), and 1/4 had a low 24-hour expectation of survival. Likewise, the mean Glasgow score on admission was 6, consistent with the types of patients included in the study, as confirmed by the fact that close to 98% of the patients were transferred to the ICU with invasive mechanical ventilation. In almost 1/2 of the patients, body mass index was classified as normal. Consistent with the risk of intracranial pressure increase, pure inhaled technique for anesthesia maintenance was not used in any of the patients. Total intravenous anesthesia was used only in 1 patient with a neoplastic posterior fossa lesion with brain stem involvement because of the need for intraoperative somatosensory evoked potentials and cranial nerve monitoring.
The use of hyperosmolar solutions was also assessed in this study. However, estimations were qualitative because data taken from the clinical record did not always allow for precise quantification of the solutions administered. Mannitol was used in a slightly greater proportion than hypertonic solutions during the surgical procedure, whereas both therapies were used in the same proportion in the ICU. These substances were used primarily to prevent or avoid cerebral edema, but in patients with hyponatremia, 3% saline solution was also frequently used as sodium replacement therapy. Again, lack of data in the clinical record prevented the analysis on sodium administration as part of the treatment in patients with hyponatremia. It is worth noting that no evidence was found in the records of hyponatremic patients of an objective calculation of the amount of hypertonic solution needed for adequate replacement using the Adrogue-Macias formula to avoid fast infusion rates that could induce overcorrection injury.17
This study found that close to 1 out of 4 patients had some degree of hyponatremia at least once during the first 5 days following the surgical procedure. Various studies have evaluated the presence of hyponatremia in these types of neurosurgical patients, with different findings depending on the neurological disorder. This study found a higher incidence of hyponatremia among patients with subarachnoid hemorrhage (66%) compared with brain tumor, head injury, and others. A series of 464 patients with this diagnosis4 found an incidence of hyponatremia of 15.6%, much lower than the incidence of 25.3% found in our research; however, in their publication, hyponatremia was evaluated on admission to hospital, whereas the patients with spontaneous subarachnoid hemorrhage included in our study were assessed after they had been taken to surgery for the management of intracranial hypertension; the more critical condition of the patients in our study implies a higher number of complications. Another study assessed the presence of hyponatremia in patients diagnosed with subarachnoid hemorrhage admitted to a neurocritical care unit and obtained an incidence of 24%, a value that is still lower than the one found in our group of patients.18 In 2006, in a retrospective study of 316 patients with subarachnoid hemorrhage, Kuramatsu et al published an incidence of 56.6%, which increased to 66% when the patients were taken to any form of intervention, either open or through neuroradiology.4 These results are much closer to the findings of our research, as is also the case with the prospective study by Hannon et al19 in 2014 which found an incidence of 49% in patients with spontaneous subarachnoid hemorrhage.
In the patients included in our study who were admitted with head injury, the incidence of hyponatremia was 20%. In 2007, a study assessed 298 patients with severe head injury and found a 16.8% incidence of hyponatremia.10 The incidence of hyponatremia in patients with space-occupying lesions was 23% in our study, similar to the incidences published in different series, that also reported a 16% incidence of hyponatremia in patients with trans-sphenoidal surgery.20,21 Of the patients included in our study, only 1 was taken to trans-sphenoidal resection ofa pituitary tumor, but this particular patient maintained normal sodium levels during the follow-up period; consequently, no comparison is possible in this setting. Similar to the reports in these studies, our research did not find a significant difference in terms of the preoperative characteristics of the patients, except for the admission diagnosis.
Several studies have mentioned the difficulty to distinguish between SIADH and CSWS.14,19,22 Among the patients who developed hyponatremia in our study, the incidence of CSWS and SIADH was 5% and 10%, respectively. Treating physicians categorized all these diagnoses as suspected, based on the presence of polyuria and increased urinary sodium. There is no documented evidence of evaluation of other parameters such as hematocrit, BUN, or volemic state, although serum osmolarity was calculated in some of them. In fact, it is worth noting that 2 patients with a probable diagnosis of CSWS did not exhibit hyponatremia and one of them actually had hypernatremia of 154mEq/L associated with polyuria and urinary sodium of 188 mmol/L at the time the diagnosis was suspected, whereas the other patient maintained sodium values within normal parameters during hospital stay, with urine output of up to 9 mL/kg/h. Both patients belonged to the severe head injury subgroup, prompting aggressive fluid resuscitation that put them in the redistribution phase at the time of the suspected diagnosis. Similarly, a patient diagnosed with SIADH had hypernatremia of 190mEq/L associated with polyuria at the time of diagnosis. In similar studies, the incidence of SIADH has been much higher than that of CSWS,23 unlike our findings. However, it is worth highlighting that these were diagnostic impressions in critically ill patients in whom hemoconcentration, glycemia, fluid balance, and changes in renal function tests may vary significantly due to multiple causes, making diagnosis even more challenging.
The first line of treatment for hyponatremia is sodium deficit replacement, and 3% saline solution has been shown to be an effective and safe therapy for increasing serum levels.24,25 However, as discussed previously, lack of data made it impossible to distinguish which patients received hypertonic solutions specifically for the management of hyponatremia and which patients received them as part of the therapy already instituted for the management cerebral edema. The use of specific pharmacological treatment that has been recommended for patients with SIADH and CSWS26-28 could not be assessed objectively because only 1 patient was prescribed fludrocortisone, whereas no patient had the indication for management with vasopressin receptor antagonists.
No significant differences were found in the sample population in terms of the demographic variables of all the patients with hyponatremia as compared with those who maintained normal sodium levels. Other studies have reported similar results, with no differences in patients with head injury who developed hyponatremia.10 On the other hand, a study conducted in 2013 found that in patients taken to trans-sphenoidal surgery, the only factor that showed a significant difference in terms of postoperative hyponatremia was preoperative hypopituitarism.20 In our study, the only finding associated with a significant increase in the incidence of hyponatremia was the diagnosis of subarachnoid hemorrhage on admission, with and 8-fold increase in the probability of this electrolyte disorder (OR 8.0; 95% CI: 1.777-36.018).
The present study found a higher proportion of patients with good neurological recovery at discharge in the group that maintained sodium levels above 135 mEq/L. Similarly, a study carried out in 2006 showed better values on the Glasgow scale in patients with severe head injury who maintained normal sodium levels. However, the results of our study were not statistically significant.
There was no difference in mortality rate between the patients with normal sodium values and those with hyponatremia, similar to the results published in 2006 and 2014 in patients with non-traumatic subarachnoid hemorrhage.29,30 In the general population, significant increases of up to 60 times have been found in patients with severe hyponatremia.1,29,31 Likewise, a 12-fold increase in mortality was reported in patients with sodium levels below 128 mEq/L as compared with those who maintained normal levels.32 In patients with neurological disorders, results have repeatedly shown higher mortality with this electrolyte alteration.2,9,33 However, the characteristics of patients included in different studies have been very heterogenous when compared with our observation, which included only critically ill patients who are already at a high risk of dying.
Limitations
One important limitation was the fact that the diagnosis of SIADH and CSWS was made on the basis of clinical suspicion and not on paraclinical testing, because the majority of patients were critically ill and criteria such as hemoconcentration, blood sugar, fluid balance, and changes in renal function tests may vary significantly due to múltiple causes, increasing the difficulty of the diagnosis. However, it is worth stating that the patients included in the study were those taken to neurosurgery and who developed hyponatremia regardless of the preceding clinical diagnosis, meaning that any prior situation or diagnostic difference would not alter the results of the study. Another limitation of our study was the fact that no target controlled infusion pumps, ideal for managing total intravenous anesthesia, are available in our region and, the most frequently used technique was, therefore, balanced general anesthesia.
Future studies should be carried out with a larger sample population to obtain statistically significant results.
Conclusion
Non-traumatic subarachnoid hemorrhage appears as a risk factor for the development of hyponatremia, with an 8-fold increase in probability. In patients taken to neurosurgery with a diagnosis of subarachnoid hemorrhage or space-occupying lesion, the development of hyponatremia suggests an unfavorable neurological outcome, with no circumstantial effect on mortality.
General mortality was high (35%) and did not change in the presence of postoperative hyponatremia. Also, survival was not affected by the severity of the hyponatremia or by the diagnosis of any of the hyponatremic syndromes studied.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.















