Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.2 Bogotá Apr./June 2014
Diagnostic Performance of, and findings from, Capsule Endoscopy for patients with Gastrointestinal Bleeding with Obscure Origins at the Clínica Universitaria San Juan de Dios in Cartagena, Colombia
Fernando García del Risco, MD. (1), Elizabeth Arrieta López, MD. (2)
(1) Clinical Gastroenterologist and Associate Professor in the Faculty of Medicine at the Universidad De Cartagena and Chief of the Gastroenterology Service at Clínica Universitaria San Juan de Dios in Cartagena, Colombia.
(2) Second year resident in Internal Medicine at the University of Sinu, Cartagena campus. MSc in Clinical Epidemiology from the Universidad Naciónal de Colombia, Clínica Universitaria San Juan de Dios in Cartagena, Colombia.
Received: 25-11-13 Accepted: 08-05-14
Abstract
Introduction: Capsule endoscopy (CE) is currently the study of choice for the exploration of Obscure gastrointestinal bleeding (OGIB) after obtaining negative results from esophagogastroduodenoscopy (EGD) and ileocolonoscopy. Objective: The objective of this study was to evaluate the diagnostic yield and findings from capsule endoscopy studies of the small intestines of 50 patients with gastrointestinal bleeding of obscure origin in our institution. Materials and methods: This was a retrospective, descriptive and observational study of a cohort of patients seen for OGIB at the Clínica Universitaria San Juan de Dios in Cartagena from January 1, 2010 to April 31, 2013. Diagnostic yields from CE were categorized as highly significant lesions (P2), insignificant lesions (P1), and normal (P0). STATA 11.0 software was used to analyze results. Results: Fifty patients suspected of having OGIB were included: 34 (68%) obvious cases and 16 (32%) cases of occult OGIB. Thirty-one (62%) of the patients were women 62%, and the mean patient age was 60.14 ± 20.02 years with an age range of 20 to 87 years. CE was performed on an outpatient basis in 26 cases (52 %) and in-hospital in 24 cases (48%). The diagnostic yield of CE for diagnosing OGIB (P2 lesions) was 58 % (29/50). It was 61.74 % (21/34) for obvious OGIB and 50 % (8/16) for occult OGIB. The preparation was suitable in 92% of the patients. Complete examination of the small intestine was achieved in 96% of the cases. Two patients retained the capsules. 51.9 % of the P2 lesions found were vascular, 20.6 % were tumors, 13.7 % were parasitic, and ulcerative lesions and inflammatory diverticula accounted for 6.9 % of the lesions each. P2 lesions which were responsible for OGIB, but which were outside of the ranges of EGDs and colonoscopy, were found in 24.13 % (7/29) of the patients. One was in a patient's esophagus, two were in patients' stomachs, one in a patient's duodenum, and three patients had them in their right colons.
Conclusion: The diagnostic yield of CE in our series was 58%. Vascular lesions (angiodysplasia) were the leading cause of bleeding which is a result that is comparable to other publications internationally and in Colombia. One striking result of our study was that the second causes of OGIB were tumors and parasitic infestations in the population studied.
Keywords
Capsule endoscopy, melena, hematochezia, anemia, gastrointestinal bleeding.
INTRODUCTION
The invention of capsule endoscopy (CE) in 2001 revolutionized the study of the small intestine (1). This noninvasive method allows exploration of the entire small intestine and obtains high-resolution images in most patients. Currently, it is considered to be the study of choice for patients with obscure gastrointestinal bleeding (OGIB) after an initial negative evaluation with bidirectional endoscopy (upper endoscopy and ileo-colonoscopy) and/or an x-ray of the small intestine (2). CE's sensitivity of 89% and specificity of 95 % are significantly higher than radiological and endoscopic diagnostic techniques such as push enteroscopy, and double-balloon enteroscopy (DBE) (3). Although DBE has a rate of detection similar to that of CE, it has the disadvantages of being an invasive endoscopic procedure that requires a long time and the use of general anesthesia. Moreover, it is not exempt from complications, and can be technically difficult to explore around the small intestine (4). CE is a minimally invasive and easy to perform procedure which patients tolerate very well. CE detection rate of lesions which are potential sources of OGIB varies between 40% and 80% (5). Significant bleeding lesions found with CE in OGIB patients include vascular lesions (angiodysplasia), tumors, ulcers, diverticulitis and parasites (5). Since the cause of OGIB is found within the range of upper and lower endoscopy in up to 25 % of the cases studied, a second look with EGD and/or colonoscopy is recommended before capsule endoscopy is done (6).
Since no studies indicating the main causes of OGIB on the Atlantic coast of Colombia have been published to date, we performed this study to begin to find the answers.
Definitions
- OGIB was defined as the presence of melena and/or hematochezia or as persistent anemia with positive occult blood test after negative EGD and colonoscopy (8, 9).
- Overt OGIB was defined as OGIB manifested by melena and/or hematochezia (9).
- Occult OGIB was defined as OGIB manifested by iron deficiency anemia or persistent blood in stool (9).
- Anemia is defined as hemoglobin <13 g/dl in men and <12 g/dl in women over 3 months without proctologic, gynecological or obvious bleeding.
Preparation
- Appropriate preparation was defined as little presence of fluids and debris, and little interference in the display of the intestinal mucosa.
- Inadequate preparation was defined as abundant presence of fluids and debris with significant interference in the display of the intestinal mucosa.
Lesions found with CE
Lesions found with CE were classified according to the degree of relevance to a definitive diagnosis of a patient:
- (P0): no lesions (normal)
- (P1): slightly significant lesions (erosions, xanthomas, lymphoid nodular hyperplasia)
- (P2): highly significant lesions (angiodysplasia, tumors, ulcers, hookworms and other parasites, diverticula)
Achievement of Diagnosis
Achievement of Diagnosis was defined as identification of P2 type lesions responsible for OGIB.
Lesions location in the small intestine
Lesions in the various segments of the small intestine were located with software that allows EC placement according to the capsule's position on a graph on a monitor. The duodenum is located just after the capsule passes the pylorus. In the upper part, the capsule moves through the intestine from right to left. The jejunum is located when the capsule is on the left side of the tracking device or in the proximal third of the section of the intestine seen on the screen. The ileum is located when the capsule is on the right side of the location device or in the distal third of the small intestine. A capsule located in the center of the abdomen or in the middle third of the small intestine was classified as a jejunoileal lesion.
MATERIALS AND METHODS
Study design
This is a descriptive and retrospective cross-sectional study of a cohort of patients who underwent capsule endoscopy of the small intestine because of OGIB at the Clínica Universitaria San Juan de Dios in Cartagena, Colombia from January 1, 2010 to April 31, 2013. Adult patients were included when they had obvious or occult OGIB and had undergone CE following negative results from an esophagogastroduodenoscopy (EGD) and a colonoscopy. Clinical records, CE records and the follow-up records were reviewed.
Preparation and study analysis
All patients included in the study were prepared according to the protocol established in our institution. The protocol calls for a liquid diet and ingestion of 2 liters of polyethylene glycol (PEG) the night before the procedure. On the day of the procedure 8 sensors were placed on the abdominal wall and connected to a data recorder housed in a belt worn by the patient during the entire procedure. After synchronization of the capsule with the data recorder, each patient ingested the capsule (PillCam© Given Imaging, Israel) in a half glass of water. The data logger was connected to a computer and the images were downloaded and processed at the workstation. The resulting video of 58,000 images was extensively studied by a trained gastroenterologist. The study duration was 8 hours. The information obtained was recorded in an Excel database (Microsoft Office 2010).
Statistical Analysis
Descriptive statistical analysis was performed for each variable. The mean and standard deviation (± SD) were calculated for each continuous variable and the median and interquartile range (IQR) was calculated for each variable with an abnormal distribution. The probability distribution of each variable was measured by the Shapiro-Wilk test. Categorical variables were grouped into absolute and relative frequencies and percentages were used for descriptive measurement. Statistically significant differences were evaluated by testing hypotheses for quantitative variables which were estimated using the Student t test. Differences corresponding to qualitative variables were estimated by the chi-square test or Fisher's exact test when the chi-square test was not particularly applicable. In all hypothesis testing, null hypotheses with type I errors (P values) of less than 0.05 were rejected. Analyses were performed using STATA 11.0. (Statistical Software: Release 11 College Station, TX. Stata Corp LP).
Ethical Issues
The study's protocol was approved by the ethics committee of the Clínica Universitaria San Juan de Dios. Confidentiality of information was guaranteed. Patients' clinical histories contained their signed consent forms authorizing capsule endoscopy. The collection of retrospective information from medical history records is considered to be "safe" research according to Resolution 8430 of 1993 of the Colombian Ministry of Health.
RESULTS
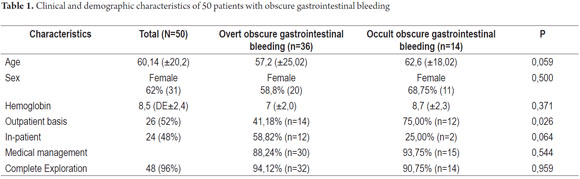
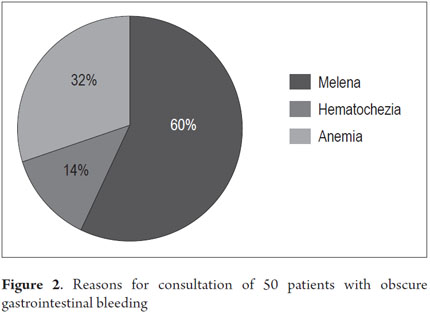
From January 1, 2010 to April 31, 2013, 59 procedures with CE were performed at the Clínica Universitaria San Juan de Dios in the city of Cartagena. Of these, 9 cases were excluded because they were conducted for reasons other than obscure gastrointestinal bleeding (Three were for recurrent abdominal pain, 3 for chronic diarrhea, two for crown disease control. One was a follow-up examination following treatment of a 4th portion duodenal lymphoma). 50 patients were analyzed, of which 55 % (34 cases) had overt OGIB and 45 % (16 cases) had occult OGIB (Figure 1). 62% (31 patients) were female. The mean age was 60.14 ± 20.02 years. Procedures in 52% of these cases were performed on an outpatient basis. The overall prevalence of anemia in the study population was 35.19 %, with a mean value of hemoglobin of 8.5gr/dl (SD ± 2.4), with a minimum value of 2.8 g/dl and a maximum of 10.8 g/dl (see Table 1). Average hemoglobin prior to the completion of the EC was 7.0 g/dl (SD ± 2) for overt OGIB and 8.7 g / dl (5.0 - 12.6 g / dl) for occult OGIB (Table 1). No statistically significant differences in baseline characteristics between patients with overt OGIB and patients with occult OGIB were found. The main reason patients consulted with a physician was melena (60 %). Anemia was the second most common reason (32%) (Figure 2). All patients had had previous endoscopic studies, and most had undergone EGDs (92 % (n = 46)) and colonoscopies (96 % (n = 48)). Four patients (7.41 %) had undergone 3 EGDs and 3.7 % of patients had undergone two or more colonoscopies (maximum of 4 colonoscopies).
Hospitalization and studies prior to CE
Four patients had been hospitalized prior to CE: three patients on 1 occasion, and one patient on three occasions.
We performed additional studies to determine the cause of OGIB on 12 patients. They included five abdominal CT scans, five intestinal transits, one scintigraphy and one angiography.
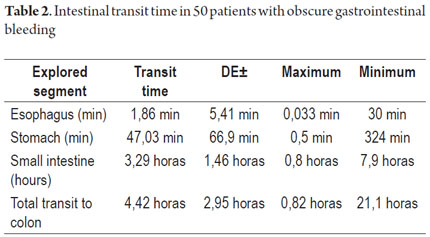
Endoscopic Capsule Transit and Incidents
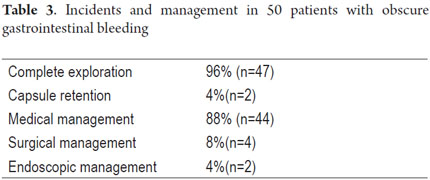
Patient tolerance of preparation was excellent. No patient reported any complaints during ingestion of the endoscopic capsule (EC). Average EC transition time through esophagus was 1.86 minute (SD ± 5.41), 47.03 minute (SD ± 66.91) through the stomach, and 3.29 hours (SD ± 1.46) through the small intestine. Total average transit time of the capsule from the mouth to the colon was 4.42 hours (SD ± 2.95) (Table 2). Complete scans of the small intestine were not possible in 2 patients (3.7 %). In one case the capsule was retained in the ileum with partial intestinal obstruction which required hospitalization and medical treatment with IV administration of corticosteroids for 3 days. The capsule was expelled after the eleventh day. In the other case the capsule was retained as the result of an ileal tumor. It was spontaneously expelled on the 4th day (Table 3).
Capsule Endoscopy Findings
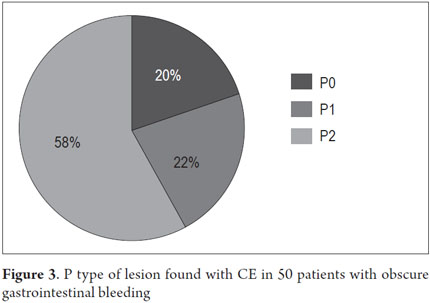
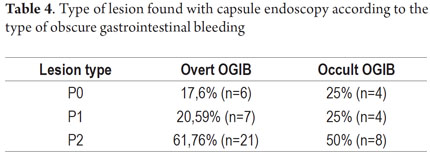
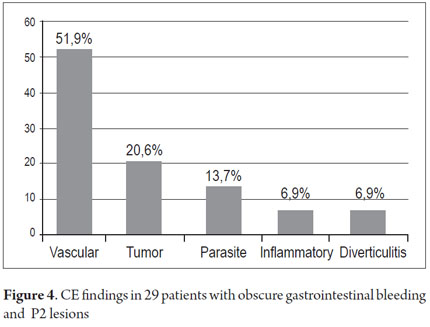
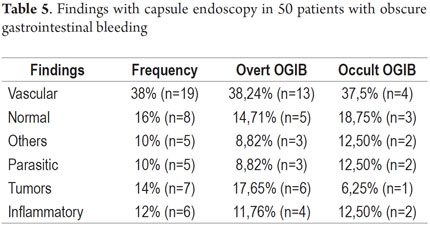
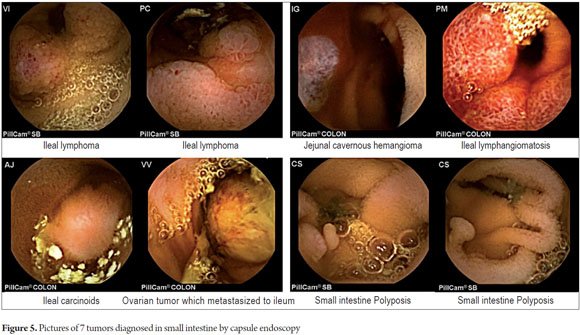
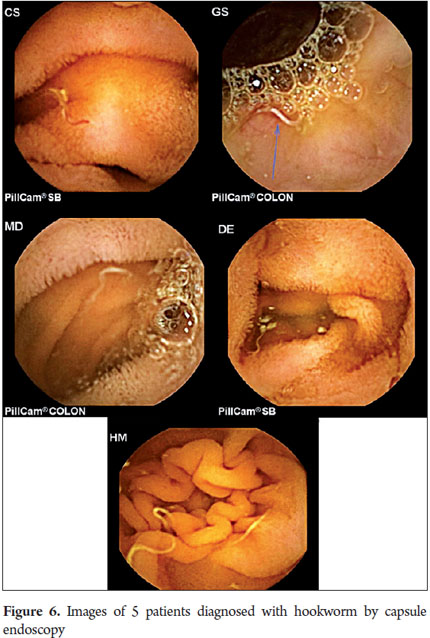
P2 lesions responsible of OGIB were found in 29 (58 %) of the 50 patients studied (Figure 3). They were found in 61.7 % (n = 21) of the patients with overt OGIB and in 50% (n = 8) of the patients with occult OGIB. Of the remaining cases, 20% of the patients (n = 10) had normal CEs (P0), and 22 % (n = 11) had no significant P1 lesions (Table 4). Frequencies of different types of P2 lesions responsible for OGIB in the small intestine were as follows: vascular 51.9 %, tumulus 20.6 %, and parasitic 13.7 % (Figure 4). Although vascular lesions were the main cause of both overt and occult OGIB, tumors were the second most frequent cause in patients with overt OGIB but in patients with obscure OGIB parasites, ulcerative and inflammatory lesions and diverticula shared the same prevalence of 12.5% in the study population (Table 5). CE allowed diagnosis of 7 tumors: two ileal lymphomas, a jejunal cavernous hemangioma, an ileal lymphangiomatosis, a carcinoid tumor, a case of small intestine polyposis and a metastatic tumor in an ovary and in the ileum (Figure 5). Five patients were diagnosed with hookworms the in duodenum and/or jejunum (Figure 6).
Anatomical locations of P2 Lesions
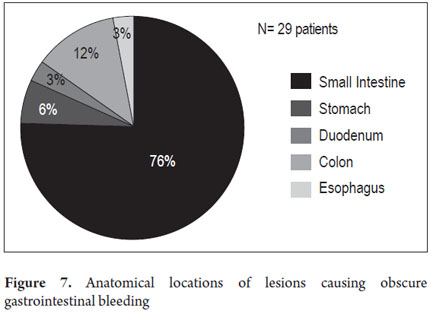
The anatomical locations of P2 lesions in the 29 patients who had them were as follows: small intestine 75.8 % (n = 22), jejunum 42.8 % (n=9) and ileum 57.14% (n=12). In 24.2 % (n = 7) of patients, P2 lesions were within reach of an EGD and colonoscopy (Figure 7) even though previous endoscopic studies had reported no abnormal findings. These cases included one angiodysplasia in the esophagus, two cases of angiodysplasia in the stomach, one case of angiodysplasia in the duodenum, and three cases of angiodysplasia in the right colon (Figure 8). CEs were normal in 8 patients (33.46 %).
P2 Lesions and Diagnostic Performance of CE
The percentage of patients with overt OGIB who were found to have P0 lesions was 17.65 %, 20.59 % had P1 lesions and 61.76 % had P2 lesions. In the group with occult OGIB, P0 lesions were found in 25% of the patients, P1 in 25% and P2 in 50% of the cases (Table 5). There were no statistically significant differences in CE's diagnostic performance, in the overall analysis (p = 0.574), or in stratification by seriousness of lesion (p = 0.723) between the two groups.
TREATMENT
Forty-four of the fifty patients studied were treated medically (oral or intravenous iron, deworming, treatment of Crohn's disease), four were treated surgically (resection of the affected bowel segment for an ileal lymphoma, a jejunal cavernous hemangioma, an ileal lymphangiomatosis and a carcinoid tumor - Table 5) and two patients underwent endoscopic balloon enteroscopy. One of these two had stenosing Crohn's disease and had an ileal stenosis dilated while the other also underwent duodenal angiodysplasia fulguration with argon plasma ablation (Table 3).
EVOLUTION
Patients were followed up by telephone and outpatient examinations. Follow-ups of 45 of the 50 patients studied were conducted for periods ranging from 3 months to 3 years and 3 months. Five patients died during follow-up: four from causes other than OGIB and one as the result of ileal lymphoma. The patient with ileal lymphoma received chemotherapy and, after two years of follow up, has no rebleeding. The patient with jejunal cavernous lymphangioma, the patient with a carcinoid tumor and the patient with ileal lymphangiomatosis were asymptomatic after one year of follow-up. (Table 6). The 34 patients treated medically for P0 and P1 lesions had no rebleeding during follow-ups ranging from 3 months to three years.
DISCUSSION
OGIB is responsible for 5 % of all cases of gastrointestinal tract bleeding. Its causes are many and varied including vascular, inflammatory, and parasitic lesions as well as tumors which are the most frequent cause. Since capsule endoscopy was invented 12 years ago, it has become an integral part of the diagnostic evaluation of OGIB (7).
This study was conducted in our center with 50 OGIB patients. It allowed us to learn about the diagnostic performance, etiology and outcome of each case. The most common indication for CE was OGIB (84.7 %). Diagnostic performance was 58%, similar to that found nationally by Juliao et al. (57 %) and Benavente in Peru (58.5 %) (8, 9). A meta-analysis by Triester showed diagnostic performance for CE of 61% to 63 % (10). A systematic review by Liao et al. of 227 published articles, found diagnostic performance of 60.5 % (5). A study by Pongprasobchai of 103 OGIB patients in Thailand found a diagnostic yield for CE of only 36%. This can be attributed to the low prevalence of vascular lesions (angiodysplasia) in Asian countries (11).
The most frequent reason for consultation of our patients was melena (53.7 %). The second most frequent reason was anemia (35.19 %) which was followed by hematochezia in 12.96 %. This is consistent with other studies (2). Patients with melena had more abnormal findings from CE (P = 0.038), but this finding was not statistically significant.
The demographic characteristics of our population were similar to those of other studies (8, 9, 10, 11). The average patient age was 60 years with female predominance in both the overt and occult OGIB groups. The average age of patients with significant findings (P2) with overt OGIB (57.5 years) was lower than that of the group of occult OGIB (62.6 years), but this age difference was not statistically significant (p: 0.059).
The leading cause of the P2 lesions which caused OGIB in our study was vascular (51.9 %) which is very similar to the primary causes found by Juliao et al. (47.1 %) (8), Galeano et al. (62.5 %) (12), and by international studies (51% to 79 %) (2, 10). It should be noted that one of the first two cases of vascular lesions (angiodysplasia) associated with OGIB ever published in our country (by Dr. Galeano) was the case of a patient referred to our institution in Cartagena (13). A study in Thailand found a low prevalence of vascular lesions, one in Indonesia found a prevalence of 27%, (11), one in India found a prevalence of 31 %, and studies in Japan with double balloon enteroscopy found prevalences of 23% and 24% (14, 15). In contrast to studies in western countries, this work showed that ulcers were the main cause of OGIB in the small intestine (41 % -53 %) in Asian countries and are followed by vascular lesions (23 % -24 %) (11).
The second most frequent cause of P2 lesions responsible for OGIB in our study were tumors (20.6 %). With a predominant anatomic location in the ileum (5/7) and in women (4/7). the high frequency tumors in our findings, when compared to other national studies and Juliao like Galeano et al. who found tumors in only 8.9% and 15.4 % of cases draws attention (8,12). The Korean Cheung study of 1,332 CE cases in 14 centers, only 57 tumors were found in SMALL INTESTINE (4.3 %), 30 malignant tumors (57%); 3 adenocarcinomas, 20 stromal tumors, 8 lymphomas and two metastatic tumors (16). We found four malignant tumors (57 %), two lymphomas, a stromal tumor, and metastatic ovarian tumor in the ileum and uncommon benign tumors (jejunal cavernous hemangioma and ileum lymphangiomatosis). Tumors that metastasize most often to the small intestine are melanoma, kidney, lung, testicular, stomach, ovarian, liver and uterine (16, 17, 18, 19). The high frequency of tumors in our study requires confirmation with rigorous follow up with a larger number of cases.
The third leading cause was parasitic diseases (13.7 %). Five patients were diagnosed with hookworm in the duodenum and jejunum which is very similar to the 10.9 % found in the study by Galeano et al. It contrasts with the study of Juliao et al. who did not find any cases of parasitosis (8). A study in India which used CE and EDB on 53 OGIB patients who lived in socioeconomic conditions very similar to ours that found infections were the cause of OGIB in 12% of their cases: 6% from hookworms and 6% from intestinal tuberculosis (20). The hookworm can be a cause of overt OGIB as has been reported by Chen et al. (21). The high frequency of hookworm infections in our study can be explained by the rural areas where sanitary conditions are poor where four of these five patients live.
The anatomical locations of the significant bleeding lesions (P2) of 9 of the 34 patients (24.2 %) were in the stomach, duodenum and proximal colon which could obviate the need for capsule endoscopy. A French study by Lepileur et al. of 911 patients found that 12.6% of the lesions found with CE were within the range of upper endoscopy and colonoscopy in 12.6 % (22), and Canadian study by Shahidi et al. found this percentage to be 17.8 %. A study by Bar Meir et al. of a series of 20 patients with occult OGIB found that a second CE showed lesions in 35% (7/20) of patients (23). For these reasons a second look is recommended before CE (24). To guarantee that lesions within the reach of an EGD or colonoscopy are seen, it is important for the endoscopist to be familiar with lesions that may go unnoticed such as Dieulafoy's lesion angiectasis, hookworms, and unusual or rare bleeding lesions. The endoscopist must also be aware that some lesions bleed slowly and/or intermittently and that the presence of blood, anemia, dehydration, use of sedatives and poor colon preparation lead to poor visualization of the mucosa (25).
Preparation in our study was adequate in 92% of the cases. In the study of Juliao et al. it was 83 % and in other series adequate preparation varies from study to study from 69% to 95 % (8, 26, 27). Although there is no single standardized preparation, diet prior to the procedure is important. We ordered a liquid diet 24 hours before the study and used 2 liters of PEG. This preparation scheme was very useful in our series. We do not use prokinetics and recommend moving the patient during the study if this is at all possible. Exploration of the small intestine was satisfactory in 96% of our cases.
Average capsule transit time from mouth to the colon was 4.42 hours (282 minutes) (SD ± 2.95) which is very similar to that reported in the study by Westeros et al. of 691 patients. That study recorded an average EC transit time from mouth to colon of 246 minutes (90-342). (28)
Capsules were retained in two patients (4%). One had Crohn's disease and the other had an ileal lymphoma. This retention rate is consistent with those described in large series. Those studies also show that patients with Crohn's disease and tumors are at the highest risk of EC retention. Hoog et al. studied 2,300 patients in Stockholm and found that the relative risk of capsule retention in patients suspected of Crohn's disease was 0.76 (CI 0.18-3.08), but was 9.39 (CI 3.32 - 26.54) in patients with known Crohn's disease and 3.88 (CI 1.18-12.81) in cases of tumors of the small intestine (29). EC retention occurs on average in 0.7 % of all patients in studies with CE and varies according to the diagnosis motivating the exam. In the general population the risk of capsule retention varies from 0.4 to 2.5 % and increases to 13 % in patients with known Crohn's disease and to 21% in patients with suspected intestinal obstruction (30, 31).
The OGIB recurrence rate during follow-up in our study was low (25 %). Positive CEs were more frequent (23 %) at 24 months follow up. The study of Juliao et al. (8). found a higher recurrence rate for bleeding (45%) at 14 months of follow up. We think that, in the 24% of the OGIB cases with P2 lesions in our study in which the causes were problems such as tumors and parasites, the treatment of these pathologies definitively resolved bleeding. This could explain the difference in recurrence rates. A Korean study by Park et al. found a rate of rebleeding of 35.3 % in 51 patients at 31.7 months follow-up (32).
Eighty-eight percent of our patients were treated medically with oral or parenteral iron and conservative actions. Eight percent underwent surgery, and four percent were treated endoscopically. In the study of Lepileur et al. of 356 OGIB patients studied with CE, 30% were managed medically and the other 70% underwent surgery, endoscopic balloon dilatation (EDB) or radiation therapy (22). This marked difference in patient management is due to several reasons: lack of interventional enteroscopists in the city, administrative problems of health care providers (medical insurance) which delayed the authorization of the studies such as CE and EDB, and referral to specialized medical centers. One of our patients with a bleeding jejunal tumor who was hospitalized for a month required more than 15 transfusions prior to CE authorization. In the study of Juliao et al., the 76% rate of medical management very similar to ours (8).
CONCLUSION
In our series, capsule endoscopy was used to diagnose OGIB in 58% of our cases which is comparable to diagnostic performances reported in other national and international studies. Particularly striking is the high frequency of tumors and parasites. This finding should be confirmed by prospective studies and a larger number of cases.
Conflicts of interest
The authors declare that they have no direct or indirect academic, scientific, financial or personnel conflicts of interest related to the publication of this manuscript.
REFERENCES
1. Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405(6785):417. [ Links ]
2. Mergener K, Ponchon T, Gralnek I, Pennazio M, Gay G, Selby W, et al. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy. 2007;39(10):895-909. [ Links ]
3. Keum B, Chun HJ. Capsule endoscopy and double balloon enteroscopy for obscure gastrointestinal bleeding: which is better? J Gastroenterol Hepatol. 2011;26(5):794-5. [ Links ]
4. Xin L, Liao Z, Jiang Y-P, Li Z-S. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc. 2011;74(3):563-70. [ Links ]
5. Liao Z, Gao R, Xu C, Li Z-S. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71(2):280-6. [ Links ]
6. Viazis N, Papaxoinis K, Vlachogiannakos J, Efthymiou A, Theodoropoulos I, Karamanolis DG. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc. 2009;69(4):850-6. [ Links ]
7. Teshima CW. Small bowel endoscopy for obscure GI bleeding. Best Pract Res Clin Gastroenterol. 2012;26(3):247-61. [ Links ]
8. Juliao Baños F, Muñoz Ortíz E, Delgado Y, Enrique C. Rendimiento diagnóstico y hallazgos de cápsula endoscópica en pacientes con sangrado digestivo de origen oscuro en el Hospital Pablo Tobón Uribe en Medellín - Colombia. Rev Colomb Gastroenterol. 2013;28(2):114-23. [ Links ]
9. Benavente Montoya M, Frisancho Velarde O. [Diagnostic yield of the endoscopic capsule and their impact in the clinical outcome]. Rev Gastroenterol Perú Órgano Of Soc Gastroenterol Perú. 2007;27(4):349-60. [ Links ]
10. Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100(11):2407-18. [ Links ]
11. Pongprasobchai S, Chitsaeng S, Tanwandee T, Manatsathit S, Kachintorn U. Yield, etiologies and outcomes of capsule endoscopy in Thai patients with obscure gastrointestinal bleeding. World J Gastrointest Endosc. 2013;5(3):122-7. [ Links ]
12. Galiano MT, Sánchez F, Pineda LF. Experiencia clínica del uso de la videocápsula endoscópica en el diagnóstico de patología del intestino delgado. Rev Col Gastroenterol. 2009;24(1):17-25. [ Links ]
13. Galiano MT, Cepeda R, García F. Angiodisplasias de intestino delgado, presentación de dos casos. Rev Col Gastroenterol. 2004;19:269-76. [ Links ]
14. Goenka MK, Majumder S, Kumar S, Sethy PK, Goenka U. Single center experience of capsule endoscopy in patients with obscure gastrointestinal bleeding. World J Gastroenterol WJG. 2011;17(6):774-8. [ Links ]
15. Ohmiya N, Yano T, Yamamoto H, Arakawa D, Nakamura M, Honda W, et al. Diagnosis and treatment of obscure GI bleeding at double balloon endoscopy. Gastrointest Endosc. 2007;66(3 Suppl):S72-77. [ Links ]
16. Cheung DY, Lee I-S, Chang DK, Kim JO, Cheon JH, Jang BI, et al. Capsule endoscopy in small bowel tumors: a multicenter Korean study. J Gastroenterol Hepatol. 2010;25(6):1079-86. [ Links ]
17. Imaoka H, Higaki N, Kumagi T, Miyaike J, Ohmoto M, Yamauchi K, et al. Characteristics of small bowel tumors detected by double balloon endoscopy. Dig Dis Sci. 2011;56(8):2366-71. [ Links ]
18. Lee B-I, Choi H, Choi K-Y, Byeon J-S, Jang H-J, Eun C-S, et al. Clinical characteristics of small bowel tumors diagnosed by double-balloon endoscopy: KASID multi-center study. Dig Dis Sci. 2011;56(10):2920-7. [ Links ]
19. Ludwig E, Kurtz RC. Tumors of the Small Intestine. In Feldman M, Friedman LS, Brandt LJ (eds). Sleisenger and Fordtrans Gastrointestinal and Liver Disease. 9th edition. Pathophysiology/Diagnosis/Management. Philadelphia: Saunders Elsevier. 2010;2145-53. [ Links ]
20. Das K, Sarkar R, Dasgupta J, Ray S, Ghatak S, Das K, et al. Obscure GI bleeding in the tropics: impact of introduction of double-balloon and capsule endoscopies on outcome. Gastrointest Endosc. 2010;72(2):292-300. [ Links ]
21. Chen J-M, Zhang X-M, Wang L-J, Chen Y, Du Q, Cai J-T. Overt gastrointestinal bleeding because of hookworm infection. Asian Pac J Trop Med. 2012;5(4):331-2. [ Links ]
22. Lepileur L, Dray X, Antonietti M, Iwanicki-Caron I, Grigioni S, Chaput U, et al. Factors associated with diagnosis of obscure gastrointestinal bleeding by video capsule enteroscopy. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10(12):1376-80. [ Links ]
23. Shahidi NC, Ou G, Svarta S, Law JK, Kwok R, Tong J, et al. Factors associated with positive findings from capsule endoscopy in patients with obscure gastrointestinal bleeding. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2012;10(12):1381-5. [ Links ]
24. Bar-Meir S, Eliakim R, Nadler M, Barkay O, Fireman Z, Scapa E, et al. Second capsule endoscopy for patients with severe iron deficiency anemia. Gastrointest Endosc. 2004;60(5):711-3. [ Links ]
25. Pennazio M. Introduction to small-bowel bleeding. Techniques in Gastrointestinal Endoscopy 2012; 14:94-99. [ Links ]
26. Koornstra JJ. Bowel preparation before small bowel capsule endoscopy: what is the optimal approach? Eur J Gastroenterol Hepatol. 2009;21(10):1107-9. [ Links ]
27. Rokkas T, Papaxoinis K, Triantafyllou K, Pistiolas D, Ladas SD. Does purgative preparation influence the diagnostic yield of small bowel video capsule endoscopy?: A meta-analysis. Am J Gastroenterol. 2009;104(1):219-27. [ Links ]
28. Westerhof J, Koornstra JJ, Hoedemaker RA, Sluiter WJ, Kleibeuker JH, Weersma RK. Diagnostic yield of small bowel capsule endoscopy depends on the small bowel transit time. World J Gastroenterol WJG. 2012;18(13):1502-7. [ Links ]
29. Höög CM, Bark L-Å, Arkani J, Gorsetman J, Broström O, Sjöqvist U. Capsule retentions and incomplete capsule endoscopy examinations: an analysis of 2300 examinations. Gastroenterol Res Pract. 2012;2012:518718. [ Links ]
30. Cave D, Legnani P, de Franchis R, Lewis BS, ICCE. ICCE consensus for capsule retention. Endoscopy. 2005;37(10):1065-7. [ Links ]
31. Li F, Gurudu SR, De Petris G, Sharma VK, Shiff AD, Heigh RI, et al. Retention of the capsule endoscope: a single-center experience of 1000 capsule endoscopy procedures. Gastrointest Endosc. 2008;68(1):174-80. [ Links ]
32. Park JJ, Cheon JH, Kim HM, Park HS, Moon CM, Lee JH, et al. Negative capsule endoscopy without subsequent enteroscopy does not predict lower long-term rebleeding rates in patients with obscure GI bleeding. Gastrointest Endosc. 2010;71(6):990-7. [ Links ]











 text in
text in