Introduction
Lithiasis of the urinary tract is one of the most frequent urological pathologies, with a prevalence that reaches up to 10% in developed countries in the general population1,2. Comparing by sex, up to 16% of men and 8% of women will develop at least one episode of renoureteral colic in their lives3. Moreover, of those patients who have presented a first episode, 11 to 39% will present a recurrence in the following 2 to 15 years, a figure that increases to 56% in patients with personal risk factors for urolithiasis4.
The prevalence and recurrence of this pathology contributes to a high socioeconomic impact associated with treatment costs and work days lost5. In the United States, in the year 2000 alone, the disease burden had an esti-mated annual cost of 5 billion US dollars, a figure that doubled in 2012 and will be estimated at 15 billion dollars by 20306-8.
Currently, the prevalence of urinary lithiasis has increased from 3.2% in 1980 to 5.2% in 1994 and to 8.8% in 20101. This increase is closely related to dietary changes including a rise in consumption of animal protein, salt, carbonated beverages and high fructose foods3,4. Also, climate change and global warming might be contributing.
Several extrinsic and intrinsic factors are associated with urinary tract lithogenesis. Among the extrinsic factors are geographical location and weather, with a higher prevalence of lithiasic disease in arid, dry, and tropical regions9. There is also an increased risk in those working in environments with high temperatures (e.g. kitchens and machine rooms) or have sedentary jobs9. Diet is another influential factor, since a high consumption of sodium, animal protein, and foods high in fructose/sucrose, as well as low water intake, all increase the risk of calculi10.
Consistent with the factors outlined previously, populations located at lower latitudes are at greater risk of urolithiasis than those at higher latitudes8. This variation in prevalence according to geographical location has been associated with higher ambient temperature and sun exposure1, causing high levels of dehydration. Furthermore, within each region there are variations in disease pattern associated to seasonal changes, presenting a higher prevalence during the summer1,8. Thus, with the rising concern of climate change, urolithiasis prevalence is predicted to increase in susceptible populations from 40% in the year 2000 to 56 and 70% for the years 2050 and 2095, respectively8.
Moreover, there are modifiable risk factors associated with over half of documented urolithiasis cases: body mass index (BMI) greater than 25 Kg/m2, daily fluid intake less than 2 liters, inadequate consumption of fruits and vegetables, and consumption of more than four servings of sugary drinks per week11. Nonetheless, these factors may be mitigated by other factors shown to have a protective effect, such as the adequate dietary intake of calcium, potassium and natural diuretic drinks such as coffee and tea12, suggesting that lithiasic disease of the urinary tract has a high potential for prevention.
Regarding intrinsic factors, the maximum incidence of urolithiasis has been reported in individuals between 20 and 60 years of age, with an average age of diagnosis of 44.8 years in men and 40.9 in women1,9. There is also a documented male predominance worldwide, with an overall ratio of 1.5-2.5 men for each affected woman8,9. However, this difference has decreased in recent years from 3.1:1 to 1.3:1 associated with lifestyle homogenization between sexes1. Moreover, an increase in metabolic syndrome, obesity and diabetes has also been shown to play an important contributing factor in both sexes1.
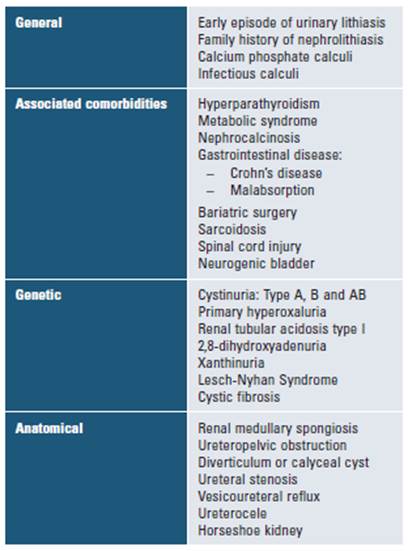
High-risk patients for this disease are defined as those having an increased chance of recurrence or growth of existing calculi. Therefore, these patients should be considered candidates for preventive pharmacologic therapy (Table 1)13. The risk of recurrence depends on both the type of stone and disease severity.
This review of the literature seeks to provide urologists with a comprehensive understanding of the metabolic implications behind urinary stone formation, as well as provide recommendations regarding its study and management beyond surgical interventions. Of note, the factors mentioned above and further detailed in the following pages, generally do not present themselves in these patients with a complete and elaborate disorder, but instead present with a combination of elements that coincide, thus creating a high urolithitic risk.
Calculi formation mechanism
The urolithiasis process begins with the nucleation of crystals and their peripheral aggregation with matrixforming proteins9. Nucleation occurs as solutes reach the point of urinary supersaturation for homogeneous nucleation, or around other structures such as crystals, cell membranes, protein aggregates and foreign bodies for heterogeneous nucleation6,7. Stone growth occurs according to the etiology of the stone; the amount of protein is inversely proportional to the probability of an underlying metabolic disorder, while the amount of matrix is in turn associated with an infectious disease6,14.
The initial histopathologic and macroscopic manifestation of crystal deposition is visualized as Randall's plaques9,15,16. These are micro- and macroscopic deposits of calcium that originate in the basement membrane of the loop of Henle, which extend into the interstitium below the urothelium of the renal papilla17,18
Types of calculi and composition
I. Calcium stones
Calcium calculi account for 80% of stones in adults 19 we performed an ambulatory metabolic protocol with diagnostic purposes. From the total sample 79% of stones were made of calcium salts (oxalate and phosphate. Its formation begins in the medullary interstitium, where the calcium phosphate molecules are deposited before passing to the papilla and forming the Randall's plaques. Subsequently, the superposition of phosphate and calcium oxalate crystals form the stones themselves17,20.
The most frequent composition of urinary stones is calcium oxalate (CaOx), present in either monohydrate (COM) or dihydrate (COD) forms, and characterized by calcium and oxalate excess in depletion of citrate and magnesium14,16. The COM calculi are characterized by a smooth surface and occur in hyperoxaluric states2) metabolic evaluation of stone formers and preventive medical therapy is underutilized. The causes for this are multifactorial. Recent technological advances, including extracorporeal shock-wave lithotripsy (ESWL, while the COD calculi are morphologically characterized by a spiculate surface and tend to occur in hypercalciuric states2,4metabolic evaluation of stone formers and preventive medical therapy is underutilized. The causes for this are multifactorial. Recent technological advances, including extracorporeal shock-wave lithotripsy (ESWL. While COD stones are more frequent in young people, the concentration of COM increases progressively with age, reaching a peak between 40 and 70 years8. The main reported risk factors for calcium oxalate stones are hyperoxaluria, hypercalciuria and hypocitraturia.
Of note, calcium oxalate and calcium phosphate stones differ in their saturation points, precipitating at acidotic and alkalotic pH, respectively. Increases of urine pH to values higher than 6.5-7.5 lead to greater conversion of monobasic to dibasic phosphate, thus increasing risk of calcium phosphate stones, particularly in the presence of hypocitraturia (described below). Moreover, patients with mixed calcium oxalate/calcium phosphate stones have lower urine citrate and higher pH when compared with calcium oxalate stone formers alone21.
II. Hyperoxaluria
Oxalate is a salt that is obtained both exogenously (directly from food intake) as well as endogenously, after hepatic metabolism of glyoxylate, glycine, hydroxyproline, and ascorbic acid22. In a healthy person, only 2 to 10% of the dietary oxalate enters the bloodstream, and the rest is used as energy source by the intestinal microbiota23,24. The dianionic oxalate is chelated by the metal cation calcium, this being the main regulator of its intestinal absorption22,25. Conditions that increase the absorption of oxalate or its production are thus predisposing factors to develop urolithiasis: dietary habits, enteric diseases and genetic conditions26.
Oxalate excretion occurs in the kidneys: the breakdown of ascorbic acid and glyoxylate accounts for 80-90% of losses, and the exogenous oxalate for the remaining 10-20%27. Urinary oxalate is directly proportional to its serum concentration (hyperoxaluria > 40 mg/day)28.
The main oxalate sources are plants and their products, especially seeds and leafs29. Although oxalate is ubicous in diet, its biodisponibility varies among different foods groups. The highest immediate rise in urinary oxalate happens after consuming spinach, while a delayed rise occurs after consuming products such as chocolate, tea, and cranberry and orange juice28.
Ethylene glycol is used primarily as a component in antifreeze and is associated with nephrolithiasis, nephrocalcinosis, renal failure and death30. Its provoked or accidental ingestion results in the production of oxalic acid that binds to calcium leading to urine oxalate crystals31.
Avoiding foods rich in oxalate, particularly spinach an rhubarb (limiting overall daily consumption to 100 mg/day), and increasing dietary calcium intake (1000-1200 mg/day), may help in the prevention of urinary tract stones associated with hyperoxaluria23.
Enteric hyperoxaluria occurs due to extensive intestinal resections with intact colon and inflammatory bowel diseases, such as Crohn's disease32 This conditions lead to bowel acidification and an increase in oxalate's permeability through the endothelium lining30,33,34) Furthermore, these diseases are associated with bacterial decolonization of Oxalobacter formigenes, a Gram negative anaerobic bacillus that resides as part of the body's normal intestinal flora and is responsible for metabolizing excess oxalate, leading to an hyperoxaluric state32,35. Also, abscense of Oxalobacter formigenes leads to greater levels of urinary calcium36.
Other oxalate-degrading bacteria include Enterococcus faecalis, Providencia rettgeri, Eubacterium lentum, Escherichia coli, Lactobacillus spp. and Bifidobacterium spp37. The extensive use of antibiotics also leads to bacterial decolonization of the gut and increases the risk of urinary litiasis37
Furthermore, primary hyperoxaluria (PHO) is a genetic disease characterized by urolithiasis and severe renal damage30. The most common variant is PHO type I, which occurs due to enzymatic damage of hepatic peroxisomes responsible for oxalate degradation, leading to excessive oxalate renal elimination and injury through nephrocalcinosis and recurrent nephrolithiasis. While PHO type II and III are less severe forms of the disease, their association to stone formation has likewise been reported30. Treatment of the cause of hyperoxaluria will prevent secondary urinary lithiasis.
III. Hypercalciuria
Hypercalciuria (> 300 mg/day) is defined as an increase in urinary calcium excretion, independent on serum calcium levels38. While, 99% of the body's calcium stores is found in the skeleton, only 1% remains in the intra and extracellular spaces39. This balance relies on the normal interplay between intestinal absorption, renal reabsorption, and bone resorption39.
Serum calcium is found in ionized form (48%), bound to proteins (46%) and in fractionated complexes (7%)39. These complexes bind to larger molecules, such as citrate and phosphate, and are thus responsible for stone formation39. Approximately 60 to 70% of the filtered calcium is reabsorbed in the proximal convoluted tubule, 20% in the loop of Henle and 10% in the distal convoluted tubule39. This last portion is the main regulatory site due to the expression of hormone-sensitive receptors, and as such, disruptions in its function is associated with nephrolithiasis39.
While calcitriol and parathyroid hormone are hypercalcemic hormones that promote bone resorption, calcitonin is hypocalcemic, and therefore stimulates bone accumulation40. Moreover, vitamin D favors calcium fixation at physiological levels and bone resorption at higher doses40,41. Primary hyperparathyroidism, prolonged immobilization, multiple myeloma, solid cancers and hyperthyroidism causes unbalanced bone remodeling, a pathological state in which resorption generates a hypercalciuric state39,41,42. Moreover, patients with vitamin D hypovitaminosis (be it from inadequate sun exposure, limited oral intake, or impaired intestinal absorption) may develop a secondary hyperparathyroid state, compounding the risk of hypercalciuria and kidney stone formation risk41,43. Management of hypercalciuria includes loop diuretics and thiazides to increase renal excretion and bisphosphonates to inhibit bone resorption39-41,44.
In patients with underlying intestinal disease and malabsorption, hypercalciuria occurs due to metabolic acidosis and intraluminal calcium sequestration42. The sequestration in turn causes hyperoxaluria30,40, increasing nephrolithiasis incidence.
Additionally, hypernatremia (> 144 mg/dL) increases calciuria due to the decrease in calcium reabsorption at the level of the proximal convoluted tubule and the loop of Henle44. As such, low-sodium diets reduce the risk of urolithiasis in patients with recurrent CaOx stones44.
I.III. Hypocitraturia
Urinary citrate binds calcium and/or phosphorus in the renal tubular lumen, decreasing the concentration of free calcium45,46. Thus, hypocitraturia (< 170 mg/day) favors the precipitation of CaOx crystals despite normal levels of urinary calcium34. The most frequent causes of hypocitraturia are intestinal malabsorption, renal failure, distal renal tubular acidosis, hypocalcemia, use of thiazides and carbonic anhydrase inhibitors (acetazolamide and topiramate), and urinary tract infections45,47).
In distal renal tubular acidosis, impaired urinary acidification leads to increased citrate reabsorption (for the formation of bicarbonate). Furthermore, as a consequence of metabolic acidosis, calcium bone resorption increases, generating a hypercalciuric and hypocitraturic environment that predisposes to CaOx calculi formation48. Moreover, a low urinary pH causes the precipitation of calcium phosphate salts, thus further increasing nephrolithiasis48.
As metabolic acidotic states in turn lead to decreased citrate excretion combined with an increased calcium and uric acid excretion, a favourable (physiologic) milieu for stone formation is produced49. This leads to the consideration of other, often overlooked, conditions associated with mild acitodic states, including (but not limited to) being overweight/obese, diabetic, and metabolic syndrome; all of which constitute intrinsic risk for nephrolithiasis49-51.
I.IV. Hypomagnesuria
In urine, magnesium ions inhibit stone formation by destabilizing CaOx molecules52. This inhibitory role (along with the action of citrate), is effective even in states of low urinary Ph52. In other words, low urinary magnesium increases free oxalate in the urine, increasing stone-formation propensity53.
Under normal conditions, 96% of the filtered magnesium is reabsorbed in the tubular system39. As such, hypomagnesuria (excretion < 50 mg/day) results directly from hypomagnesemic states. The main causes of hypo-magnesemia include gastrointestinal losses (chronic diarrhea and chronic use of proton pump inhibitors) and renal losses (use of loop diuretics, uncontrolled diabetes, hypercalcemia and alcohol consumption)39,54-56.
I.V. Hyperphosphatemia
Hyperphosphatemia, through the formation of Randall's plaques, is a risk factor for the formation of calcium stones39. The body's equilibrium of phosphorus is determined by the balance between the excretion of phosphate and the dietary intake39. Once in plasma, the phosphate is either transported into the intracellular space or stored in the skeletal system39. In this way, the regulation of this ion is closely related to calcium concentrations: parathyroid hormone decreases renal reabsorption of phosphate in the proximal convoluted tubule and causes phosphaturia39. The management of hyperparathyroidism includes phosphate binders, vitamin D analogues, and calcimimetics39.
II. Uric acid stones
Hyperuricemia is classified as primary and secondary, and in turn, in hyperproduction and uric acid hypoexcretion24,57. In the lithiasic patient, hyperuricosuria may occur due to elevated or normal levels of uric acid in the blood. When associated with hyperuricemia, it is suggestive of gout and metabolic alterations, whereas associated with normouricemia is suggestive of purine-rich diets24,57. The hyperuricosuria with normouricemia resolves once the consumption of red meat, alcohol and seafood has decreased.
In addition, hyperuricosuria (> 990 mg / day) causes greater elimination of uric acid decreasing urinary pH and triggering the formations of stones nuclei24,57. A pH lower than 5.7 increases the precipitation of CaOx crystals and the aggregation of these on crystals of uric acid, forming stones24,57,58. In addition, it has been described that insulin resistance, associated or not with metabolic syndrome, directly affects the ammoniogenesis and the ammonium that previously worked as a urinary buffer, will now acidify it59 in the normouricemic, normouricosuric patient. Of particular importance in this group, are those patients consuming excess animal protein and specialized diets (e.g. Atkins, keto and other low-carbohydrate/high-protein diets), as animal protein has been shown to boost urinary excretion of oxalate, which then combines with calcium and other compounds to form kidney stones60.
The treatment is based on urinary alkalinization with potassium citrate and the decrease of uric acid levels from the restriction of purine-rich animal proteins58 due to the high capacity of urinary acidification they possess59 calcium phosphate (US-CaP, and allopurinol61.
III. Struvite stones
Struvite stones are composed of phosphate crystals hydrated by magnesium ammonium and calcium apatite57. These stones are characterized by rapid growth over a period of weeks to months, forming staghorn calculi that occupy the space of the collecting system and thus often obstructing the urinary tract57,61,62 Urine usually maintains low concentrations of ammonium phosphate, however, an alkalinization of urinary pH leads to lower phosphate solubility9,57,62 and, thereof, a greater urinary availability. This happens because as urease-producing bacteria, such as Proteus spp., Klebsiella spp., and Pseudomonas spp. colonize the urinary tract57, urea is metabolized into ammonia and combines with water to form ammonium and thus increases urine Ph63. The treatment includes surgery and acetohydroxamic acid (reversible inhibitor of urea), which prevents the crystallization of struvite and apatite carbonate2.
IV. Cystine stones
Cystine is an amino acid that, due to its insolubility at normal urinary pH, precipitates to form crystals in patients with cystinuria2,64. Cystinuria is a genetic disease of autosomal recessive inheritance, characterized by the inadequate reabsorption of cystine and other dibasic amino acids in the proximal tubule of the nephron and in the gastrointestinal epithelium, causing recurrent urolitiasis64.
This condition has an overall incidence of 1 per 7000 live births and corresponds to 1% of urinary tract stones in adults65. The majority of cases of cystinuria are caused by mutations in two genes that code for subunits of the cystine transporter (SLC3A1 and SLC7A9)64. The treatment consists mainly in preventing the formation of stones through adequate hydration, dietary restriction of foods rich in methionine (e.g. meat, pork and dairy), decreased salt intake, urinary alkalinization and drugs with cystine reuptake (e.g. thiols)2,64.
Metabolic studies and calculi analysis
After complete resolution of renal colic, a 4- to 6-week waiting period (during which the patient is expected to resume habitual diet) is recommended prior to proceeding with the metabolic study66. Metabolic study should include the following aspects: medical history directed to the underlying pathology, nutritional evaluation, and urine and blood studies.
The clinical history includes the following:
Relevant comorbidities: diabetes mellitus, hyperuricemia, sarcoidosis, hyperparathyroidism, osteoporosis, inflammatory disease or intestinal malabsorption, and previous history of urinary lithiasis (and number of episodes).
Pharmacological background: consumption of topiramate, acetazolamide, thiazide diuretics, vitamin C or vitamin D supplements, antacids, and proton pump inhibitors.
Family history of calculi.
Surgical history that includes intestinal resections and bariatric surgery.
Numerous studies link the growing urolithiasis incidence to BMI. Dietary modifications are essential and a comprehensive nutritional history must be undertaken in order to identify dietary elements which contribute to stone pathogenesis10. As mentioned previously, increased body weight is associated with mild metabolic acidosis, increasing stone formation risk and thus necessitating additional nutritional recommendations to promote weight loss in overweight to obese patients49.
Nutritional appraisal quantifies daily consumption of lithogenic food components, such as sodium, sugar, animal protein (e.g. purines and uric acid) and oxalate. Likewise, protective factors must be quantified including calcium, water, fruits and vegetables67. Once dietary risk factors are identified, targeted nutritional recommendations may be endorsed.
General dietary recommendations should be considered in every patient, including:
Increase liquid intake (at least 3 Liters/day to void 2-2.5 Liters/day)
Dietary calcium: 1000-1200 milligrams/day
Fiber-rich diet (increase fruits, vegetables, and whole-grain ingestion)
Include citrate-rich food sources into diet (e.g. citric fruits)
Decrease consumption of oxalate-rich foods (e.g. spinach)
Limit salt intake to 5 grams/day or its equivalent in sodium (2 g/day), including added nitrates to most processed food
Limit daily animal protein consumption to 1 gram per patient's ideal weight (in kilograms).
A thorough metabolic study should include two 24-hour urine collections, blood analysis and physicalchemical analysis of the stone. X-ray diffraction and infrared spectroscopy by Fourier transformation are the recommended tests for the physical study of the calculi59,68. These methods permit correct identification of the type of calcium stone present and other associated stone components, such as cystine, xanthine, uric acid, urates, struvite, proteins, lipids, and/or drugs2,6.
Conclusions
Urinary lithiasis can be a recurrent disease in patients with risk factors causing high costs to the healthcare system. Is important to look for the etiology of the calculi to focus treatment on the prevention of new episodes. All physicians and urologists should counsel patients with the general recommendations and according to their metabolic studies and calculus analysis.