Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Acta Biológica Colombiana
Print version ISSN 0120-548X
Acta biol.Colomb. vol.19 no.3 Bogotá Sept./Dec. 2014
https://doi.org/10.15446/abc.v19n3.42334
Artículo de investigación
http://dx.doi.org/10.15446/abc.v19n3.42334
HISTOLOGICAL DESCRIPTION OF THE REPRODUCTIVE SYSTEM OF MALE AND FEMALE HATCHLINGS OF THE MAGDALENA RIVER TURTLE (Podocnemis lewyana)
Descripción histológica del sistema reproductivo de machos y hembras de la tortuga del Río Magdalena (Podocnemis lewyana)
ANDRÉS C. SÁNCHEZ-OSPINA1, MV; BERARDO RODRIGUEZ1, Ph. D.; CLAUDIA P. CEBALLOS2, Ph. D.
1 Grupo Quirón. Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias, Universidad de Antioquia. Medellín, Colombia. acsovet@gmail.com; birdo77@yahoo.com
2 Grupo GaMMA. Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias, Universidad de Antioquia. Medellín, Colombia. claudiaceb@gmail.com
Author for correspondence: Claudia Ceballos, claudiaceb@gmail.com
Received February 20th 2014, first decision May 14th 2014, accepted June 5th 2014.
Citation / Citar este artículo como: SÁNCHEZ-OSPINAAC, RODRIGUEZ B, CEBALLOS CP. Histological description of the reproductive system of male and female hatchlings of the Magdalena River turtle (Podocnemis lewyana). Acta biol. Colomb. 2014;19(3):427-435.
ABSTRACT
In this study we described the macroscopic and microscopic histology of the reproductive system of male and female Podocnemis lewyana neonates (3.5 months old). We found macroscopic differences in the morphology of the gonads between the sexes, with ovaries being twice as long as testes, and testes being twice as wide as ovaries. Microscopically, we identified several immature elements, such as the lack of a muscle layer in the oviduct of females, and simple epithelia instead of pseudostratified epithelia in the oviduct and epididymis described in reptile adults. We also found a black pigment observed macroscopically in the mesovarium, and macroscopically and histologically in the epididymis. This pigment is consistent with the center of melano-macrophages described in other vertebrates. Finally we described a supporting mesenchymal structure, the appendage of the oviduct, which was much longer than what has been described in other Podocnemis species.
Keywords: epididymis, histology, ovary, oviduct, pigment, testis.
RESUMEN
En este estudio se realizó una descripción macro y microscópica del sistema reproductivo de tortugas machos y hembras de neonatos de Podocnemis lewyana de 3,5 meses de edad. Se hallaron diferencias macroscópicas en las gónadas, esto es, el ovario fue el doble de largo relativo al testículo, pero el testículo fue el doble de ancho relativo al ovario. Microscópicamente se identificaron varios elementos inmaduros tales como la ausencia de una capa muscular reportada en el oviducto de hembras de reptiles adultas, y epitelios simples en lugar de epitelios pseudoestratificados en oviducto y epidídimo en reptiles adultos. También se describe un pigmento negro observado macroscópicamente en el mesoovario de las hembras, y macroscópica e histológicamente en el epidídimo de los machos. Este pigmento es compatible con los centros de melanomacrófagos reportados en otros vertebrados. Finalmente, se describe también una estructura mesenquimatosa de soporte que identificamos como el apéndice del oviducto, la cual fue mucho más larga que las descritas en otras especies de Podocnemis.
Palabras clave: epidídimo, histología, ovario, oviducto, pigmento, testículo.
INTRODUCTION
The Magdalena river turtle, Podocnemis lewyana (Chelonia: Podocnemididae), is a highly aquatic river species and endemic to the Magdalena and Sinú River drainages of Colombia (Páez et al., 2009). The adults exhibit female-biased sexual size dimorphism, with females reaching up to 50 cm and males 36 cm of linear carapace length (Páez et al., 2012). The sex of this species is determined by the temperature at which eggs are incubated (Páez et al., 2009). This is, the pivotal temperature of 33.4 °C produces both sexes in similar proportions, while higher temperatures produce mainly females and lower temperatures produce mainly males (Páez et al., 2009). Its reproductive cycle has not been studied in detail, however it is known that there are two nesting seasons: between December and March, and between June and August (Gallego-García and Castaño-Mora, 2008; Ceballos et al., 2014). Its average clutch size varies between 20 to 22 eggs, with an egg weight varying between 18-38 g and an egg size between 31-50 mm long x 21-44 mm width (Páez et al., 2012; Ceballos et al., 2014). Whether P. lewyana nests in one or both nesting seasons is unknown (Páez et al., 2012).
While these aspects of the reproductive biology have been studied, the anatomy and histology of its reproductive system has not been reviewed for this species. Overall, the reproductive system of the male turtles is macroscopically formed by a pair of testes, each with its epididymis, ducts deferens, suspensory ligaments, and a penis (Wyneken, 2001). The testes, as is the case for ovaries, are derived from the germinal crest during the embryonary period. They are located posterior to the coelomic cavity and ventral to the kidneys, and are characterized by its smooth surface and fusiform shape (Kuchling, 1999; Jacobson, 2007). Histologically, the testes are formed by a series of seminiferous tubules with germ cells developing into sperm cells or spermatozoa. When the turtle reaches sexual maturity these sperm cells enter into a series of smaller ducts, known as the rete testis, which then move into the epididymal ducts, and then into the duct deferens towards the penis (Gribbins et al., 2003; Holmes and Gist, 2004; Pagliarini Cabral et al., 2011).
On the other hand, the reproductive system of females is formed by a pair of ovaries, an oviduct, and the suspensory ligaments. Histologically, the ovary is an elongated structure formed by a cortex and a medulla. The cortex is formed by germinal beds and multiple developing follicular structures (Yntema, 1981; Callebaut et al., 1997; Hei et al., 2010; Pérez-Bermúdez et al., 2012). Germinal beds contain oogonia (primordial oocytes) and oocytes (Pérez-Bermúdez et al., 2012), which later will become a mature ovum to be fertilized when the female reaches sexual maturity. The ovarian follicular development includes three different stages: folliculogenesis, previtellogenesis and vitellogenesis, in which the oocyte increases its size gradually (e.g. from 19.2 µm to 24.9 mm in the hawksbill turtle, Eretmochelys imbricata, Pérez-Bermúdez et al., 2012). Deeper in the ovary is the medulla, which is composed of lymphoid lacunae, blood vessels, fibroblasts, collagen fibers, and smooth muscle (Callebaut et al., 1997; Hei et al., 2010; Pérez-Bermúdez et al., 2012). The size of the ovaries varies with age and the physiological state of the female, and follicles can develop in both ovaries at the same time (Callebaut et al., 1997). The oviduct is a tubular structure formed by three layers: the inner layer is formed by ciliated epithelium and connective tissue with glands and blood vessels; the middle layer or myometrium is formed by smooth muscle; and the outer layer is a serosa (Palmer and Guillette, 1988; Sarkar et al., 1995). Finally there are the suspensory ligaments that include the mesovarium, a connective tissue that fixes the ovaries to the coelomic cavity. Gonadal differentiation is closely linked to the egg incubation temperature. Specifically, low temperatures are correlated with low levels of estrogens production by means of the aromatase enzime activity that converts androgens in estrogens (Pieau et al., 1998). This is important because transitional incubation temperatures that produce both sexes (not 100 % males, or 100 % females) have been correlated with a sufficient aromatase production to induce the formation of female elements (e.g. ovarian cortex) but not enough to inhibit the development of testicular cords in testes (Pieau et al., 1998). Such gonads are classified as ovotestes at hatching, which in most cases evolve as testes. For this reason it is recommended to sex turtles by gonads histology using older hatchlings when the gonad development is better defined.
The anatomy of the reproductive system of some Podocnemis turtles has been described. For example, Hernández-Henao et al., (2013) reviewed macro and microscopically the urogenital system of P. unifilis adult males and females, and overall they found it similar to that of the reptiles in general. The reproductive system of P. expansa and P. unifilis neonates were also histologically described (Malvasio et al., 2012). In this case, authors described the primordial follicles, a remnant of the mesonephros, and the oviduct formed by low cylindric epithelium and fibrous connective tissue. This connective tissue is additionally projected to form a remnant of an appendage, or saccular structure, of the oviduct. The parenchyma of the testes exhibits the rete testis with simple cubic epithelium with some spermatogonia, and the epididymis with ciliated cubic epithelium (Malvasio et al., 2012). The basic anatomy of all reptiles is very similar, however there are variations in the accessory structures in some taxa (Norris and Lopez, 2010). Given that the Magdalena River turtle, Podocnemis lewyana, is an endangered species of Colombia (Castaño-Mora, 2002), and that the histology of its reproductive system has not been described, herein we contribute to the knowledge of the anatomy and histology of its reproductive system. We specifically provide a macro and microscopic description of the reproductive system of male and female neonates of 3.5 months of age.
MATERIAL AND METHODS
Hatchlings morphometry
This study used ten males and ten female hatchlings between 3 and 3.5 months of age collected in the Rio Claro Cocorná Sur, near the town of Estación Cocorná, Puerto Triunfo, Antioquia. Sexing these hatchlings was not possible using laparoscopy because of the small size of the gonads (<5 mm) and the turtles themselves. These turtles originated from four different clutches previously incubated at constant temperatures are expected to 100 % males: 29 °C (n=5) and 31°C (n=5), and 100 % females: 34 °C (n=10) (Páez et al., 2009).
Hatchlings were maintained in an outdoor pool for 3.5 months with food ad libitum as part of the headstarting program leaded by the non governmental organization Asociación Ambientalista Futuro Verde, AAFUVER (Romero, 2011). With the purpose of testing for sexual size dimorphism at this early age, we also monitored body growth. Specifically, we measured egg weight and body weight at seven days and 3.5 months of age using a Lexus balance to its nearest 0.1 g. We used an analysis of variance (ANOVA) to test for sexual dimorphism in body weight.
Tissue collection, dissection and staining
When turtles were 3.5 months (juveniles) were euthanized by a lethal injection of 0.1 ml (1 mg) of propofol (Carpenter, 2006) in the occipital venous sinus. Because turtles can withstand long periods of anoxia, following the injection turtles were decapitated to ensure death (McArthur et al., 2004). After the coelomic cavity was exposed, the digestive tract was removed to expose the reproductive system including the kidneys that are intimately connected. Tissues were temporally fixed in 70 % alcohol, and then the samples were sent to the animal Pathology Lab at the Veterinary School of the University of Antioquia were the samples were transferred to 10 % buffered formaldehyde.
Once in the lab the organs were finely dissected using a stereoscope, a scalpel blade No. 4, and a Backhaus dissecting forceps to separate the gonads from the kidneys and the peritoneum. Tissues were embedded in paraffin, cut with a microtome, stained with haematoxylin and eosin, and mounted on a slide with Canada balsam. To identify specific elements in the mesenchyme we also used Periodic acid-Schiff (PAS) and Masson's trichrome (García del Moral, 1993).
RESULTS
Early sexual dimorphism
We did not find sexual dimorphism in egg weight (p=0.3294) or hatchlings body weight at seven days of age (p=0.2158). In contrast, body weight at 3.5 months of age was sexually dimorphic (p=0.0009) with females being about 20 % heavier than males (Table 1).
Macroscopic male´s reproductive system
The reproductive system of P. lewyana males is identified by a pair of testes and their epididymides. Macroscopically these have an elongated morphology, measuring 8 mm long x 4 mm wide x 2 mm deep (Fig. 1A). Its coloration in vivo is transparent-white, but changes to chalky white after fixation. The epididymis is observed in its distal portion, dark colored, and of approximately 3 mm long.
Microscopic male´s reproductive system
Histologically, the entire reproductive system (Fig. 2A) is coated by a single layer of flat and fusiform cells. These cells have a pattern of dense to granular chromatin, and are supported by a connective tissue. The tubular compartment of the male reproductive system is composed by the epididymis and testicle, while the interstitial compartment is overall composed of connective tissue.
The epididymis (Fig. 2B) is composed of several ducts with cuboidal to almost columnar ciliated epithelium. These cells have rounded to ovoid nucleus with granular chromatin pattern, a dark basophilic nucleolus, and a cytoplasm with eosinophilic cilia. It was also observed a scattered black pigment located both at the interstitial and intracytoplasmic level of the epididymis. The interstitial compartment of epididymis has a connective tissue and multiple cells with spindle morphology, likely fibroblasts. In the basal portion of the epididymis there is a fibrovascular connective tissue that connects it to the testicular parenchyma.
The testicle (Fig. 2C) is formed by an external capsule that enters the organ and divides it by connective tissue septa, while its parenchyma is formed by rounded seminiferous tubules. These tubules are formed by low cuboidal epithelium with spermatogonia and sustentacular or Sertoli cells. The spermatogonia are large cells with a low relationship nucleus: cytoplasm (small nucleus compared to the cytoplasm), with a round, small and central basophilic nucleus. The sustentacular or Sertoli cells have an ovoid to spherical shape with prominent nucleoli (up to two per cell), and clear basophilic staining. The interstitial compartment of the testicle is a connective tissue composed of fibroblasts, lymph vessels, and blood vessels of small and medium caliber. There are also fusiform cells resembling myoepithelial cells with dark basophilic and dense chromatin pattern surrounding the seminiferous tubules. In the testicular stroma there is fibrovascular connective tissue composed of medium caliber blood vessels and loose connective tissue.
We also observed two more structures. The first one is a rounded structure of tubular morphology composed of ducts of low cuboidal epithelium, and its cells exhibit an spherical nucleus and a single nucleolus. (Fig. 2D-a). In its interstitial level there is connective tissue surrounded by spindle cells. The second is a mesenchymal structure of elongated morphology with similar dimensions to the epididymis (Fig. 2A-j). This organ is filled with abundant fibrovascular connective tissue, blood vessels of small and medium caliber, and spindle cells.
Macroscopic female´s reproductive system
The reproductive system of the P. lewyana female is composed of a pair of ovaries, two oviducts and a mesovarium (Fig. 1B). The ovary is characterized by having an elongated tubular morphology, measuring 20 mm long x 2 mm x 2 mm. Its coloration in vivo is transparent to white, but after fixation it turns chalk white. Parallel to the ovary we observed the mesovarium as a connective tissue that in some portions was black colored.
Microscopic female´s reproductive system
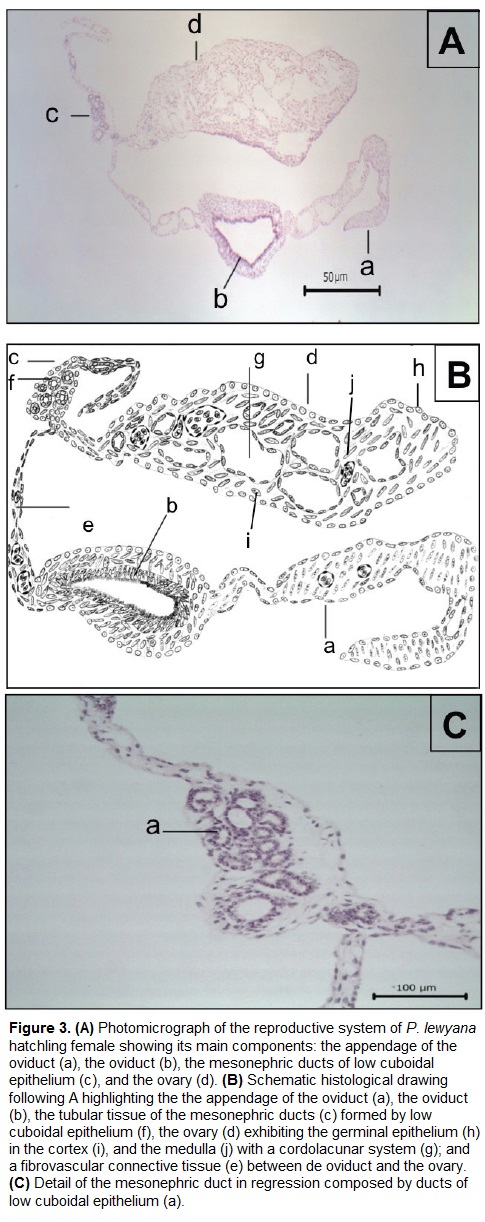
Histologically, the entire reproductive system (Fig. 3) is coated by a single epithelial layer of flat and fusiform cells. These cells have a pattern of dense to granular chromatin, and are supported by a connective tissue.
The ovary (Figs. 3Ad, 4) is coated by a thin external capsule composed of connective tissue. We observed the cortex and the medulla (Fig. 4A). The cortex is formed by a germinal epithelium made of simple cubic epithelium, which in some parts stratifies to form germinal beds (Fig. 4B) that contains immature follicular cells. Deeper in the cortex these cells are observed in a more developed stage, that form previtellogenic follicles (Fig. 4C). The oocyte inside the follicle has a low relationship nucleus: cytoplasm. The nucleus is central and rounded, and exhibits a dense chromatin pattern. The oocyte is surrounded by the ooplasm, which exhibits fibrillar morphology. The medulla of the ovary on the other hand is formed by a cordolacunar system delimited by a simple flat epithelium, connective tissue and blood vessels.
The oviduct is composed by an epithelial mucose layer (Fig. 5Aa) formed by low cylindrical ciliated epithelium composed of elongated cells, and light basophilic nucleus with a granular chromatin pattern. The cytoplasm is scarce but projected apically into the lumen forming eosinophilic cilia. Just below the epithelium there is an acellular basement membrane (Fig. 5Ab) colored reddish violet with PAS staining. The submucose layer (Fig. 5Bc) is composed by a mesenchymal connective tissue that was evidenced with Masson´s trichrome stain (Fig. 5B). This layer is composed by a lax connective tissue (Fig. 5Bc) and a dense irregular tissue layer (Fig. 5Bd) composed of collagen fibers and fusiform fibroblasts. We also observed that part of the mesenchymal component of the submucose layer has an elongated projection showing small-caliber blood vessels and connective tissue, which is surrounded by a serosa layer (Fig. 3Be).
Like in the male, we also observed two additional structures in the female. The first one (Fig. 3Ac) is located between the ovary and the oviduct. This tissue has tubular morphology and rounded structures with simple cubic epithelium, and a basophilic and spheroid nuclei exhibiting prominent nucleoli. In the interstice there are spindle cells with a granular to reticular chromatin pattern and connective tissue surrounded by fibroblasts. The second structure (Fig. 3Aa) is located near to the oviduct, and is composed of mesenchymal tissue. Three portions of fibrovascular connective tissue are differentiated, with the proximal portion being the narrowest, and the middle having the small caliber blood vessels coated by vascular endothelium. All three portions are formed by spindle cells with elongated granular chromatin and dark basophilic nucleolus.
DISCUSSION
Sexual dimorphism
In this study we observed that the ovary was twice longer than the testis, but the testis was twice wider than the ovary. In contrast, in other congeneric species such as Podocnemis expansa and P. unifilis (Malvasio et al., 2012), the gonads of hatchlings of the same age (3 months) were sexually dimorphic only in their width. Nevertheless, this finding is in agreement with the sexual dimorphism found in their body weight.
Histology
The germinal cells observed in testes and ovaries of P. lewyana at 3 months of age were in an immature stage as expected. We observed spermatogonia in the seminiferous tubules, but not spermatids or spermatozoa. Likewise, we observed primordial follicles in the germinal beds of the ovarian cortex, with an oocyte of approximately 20 µm (Fig. 4Cf), surrounded by flat follicular cells. Such development was classified as stage I of folliculogenesis in juvenile hawksbill sea turtles (Pérez-Bermúdez et al., 2012).
On the other hand, the tubular structures of males and females were also found in an immature stage. We did not observe the smooth muscle layer described in the oviduct of adult P. vogli (Hernández-Henao et al., 2013), and other reptiles such as geckos (Saltvarius wyberba) (Girling, 2002). The epididymis exhibited a simple high cuboidal ciliated epithelium, and the oviduct exhibited a simple low cylindric ciliated epithelium. In adult P. vogli these epithelia are not simple or cuboidal but cylindric and pseudostratified (Hernández-Henao et al., 2013).
A new finding was the black pigment found in the epididymis and the connective tissue that supports the ovary. Histologically, this pigment (Fig. 2B) is compatible with the melano-macrophage centers (MMC), which are aggregations of macrophages with melanin, lipofuscin and hemosiderin. These MMC have been found in spleen, liver, and gonads of other vertebrates such as fish, frogs and even Kinosternon turtles (Ravaglia and Maggese, 1995; Christiansen et al., 1996; Agius and Roberts, 2003; Pérez, 2010). Among the functions of MMC are antibacterial protection and phagocytosis, and their amount can vary with the external environment including contamination (Pérez, 2010), but also with nutrition and hormones according to the reproductive cycle (Agius and Roberts, 2003). In K. flavescens turtles MMC increased linearly with age, thus it is interesting to find MMC in P. lewyana only 3 months old.
On the other hand, the tubular structures are compatible with remnants of the mesonephric duct in the males (Fig. 2Da), and remnants of the paramesonephric ducts in the females (Fig. 3C). These ducts originate from the mesonephros or the embryonic kidney, during the early embryonic stages. While both ducts are developed in both sexes, only the mesonephric ducts remain and differentiate into the epididymis in the male and the paramesonephric duct regresses. In the female, only the paramesonephric ducts remain and develops into the oviduct while the mesonephric duct regresses (Lombardi, 1998).
The mesenchymal structures observed in males (Fig. 2Aj) and females (Fig. 3Aa) are compatible with remnants of the appendages of the oviduct. This structure was also observed in P. expansa y P. unifilis females (Malvasio et al., 2012). Although this structure has similar histologic components in all Podocnemis species, its size is different. This appendage was approximately one third of the length of the oviduct in P. unifilis, similar length in P. expansa, but twice in P. lewyana females (this study). In P. lewyana males it was as long as the epididymis. Because its histological components resemble a peritoneum we believe this structure may give support to the reproductive system within the coelomic cavity.
CONCLUSION
This is the first histological review of the P. lewyana reproductive system at this early age, however it is important to undertake future studies at older ages that may also serve as a point of comparison for pathological studies. Very importantly, we also encourage future studies on its reproductive cycle to elucidate its biological capacity to increase population size and overcome the different threats that this species is currently facing.
ACKNOWLEDGEMENTS
We thank C. Gómez-Saldarriaga for providing the specimens used in this study. Funding for histological lab analyses was provided by the Veterinary School of the University of Antioquia, and the 2013-2014 Código E01727 Sustainability Program of the University of Antioquia to C. P. Ceballos. This work was carried out under protocol approved by the Animal Experimentation Ethics Committee of the University of Antioquia in Act 76, and research permit N#° 134-0067 issued by the local government authority CORNARE.
REFERENCES
Agius C, Roberts RJ. Melano-macrophage centres and their role in fish pathology. J Fish Dis. 2003;26(9):499-509. DOI: 10.1046/J.1365-2761.2003.00485.X. [ Links ]
Callebaut M, VanNassauw L, Harrisson F. Comparison between oogenesis and related ovarian structures in a reptile, Pseudemys scripta elegans (turtle) and in a bird Coturnix coturnix japonica (quail). Reprod Nutr Dev. 1997;37(3):233-252. DOI: 10.1051/rnd:19970301. [ Links ]
Carpenter JW. Formulario de animales exóticos. 3#ª edición Buenos Aires: Intermedica; 2006. p. 540. [ Links ]
Castaño-Mora OV. Libro Rojo de Reptiles de Colombia. Bogotá, D.C., Colombia: Instituto de Ciencias Naturales, Universidad Nacional, Ministerio del Medio Ambiente, Conservación Internacional-Colombia; 2002. p. 160. [ Links ]
Ceballos CP, Romero I, Gómez-Saldarriaga C, Miranda-Ríos K. Reproduction and conservation of the Magdalena River turtle (Podocnemis lewyana) in the Claro Cocorná Sur River, Colombia. Acta Biol Colomb. 2014;19(3):393-400. [ Links ]
Christiansen JL, Grzybowski JM, Kodama RM. Melanomacrophage aggregations and their age relationships in the Yellow Mud Turtle, Kinosternon flavescens (Kinosternidae). Pigment Cell Res. 1996;9(4):185-190. DOI: 10.1111/j.1600-0749.1996.tb00108.x. [ Links ]
Gallego-García N, Castaño-Mora OV. Ecology and status of the Magdalena River turtle, Podocnemis lewyana, a Colombian endemic. Chelonian Conserv Biol. 2008;7(1):37-44. [ Links ]
García del Moral R. Laboratorio de Anatomía Patológica. Madrid, España: McGraw-Hill-Interamericana; 1993. P. 652. [ Links ]
Girling JE. The reptilian oviduct: a review of structure and function and directions for future research. J Exp Zool. 2002;293(2):141-170. [ Links ]
Gribbins KM, Gist DH, Congdon JD. Cytological evaluation of spermatogenesis and organization of the germinal epithelium in the male slider turtle, Trachemys scripta. J Morphol. 2003;255(3):337-346. DOI: 10.1002/jmor.10069. [ Links ]
Hei N, Yang P, Yang Y, Liu J, Bao H, Liu H, Zhang H, Chen Q. Fine structural observation on the oogenesis and vitellogenesis of the Chinese soft-shelled turtle (Pelodiseus sinensis). Zygote (Cambridge, England). 2010;18(2):109-20. DOI: 10.1017/s0967199409990116. [ Links ]
Hernández-Henao JW, Rodríguez-Pulido JA, Astwood-Romero JA. Anatomía macroscópica y microscópica del sistema urogenital de la tortuga sabanera Podocnemis vogli Muller, 1935 (Testudines, Pelomedusidae). Orinoquia. 2013;17(1):120-133. [ Links ]
Holmes HJ, Gist DH. Excurrent duct system of the male turtle Chrysemys picta. J Morphol. 2004;261(3):312-322. DOI: 10.1002/jmor.10251. [ Links ]
Jacobson ER. Overview of Reptile Biology, Anatomy, and Histology. In: Jacobson ER, editor. Infectious Diseases and Pathology of Reptiles. Color Atlas and Text. Boca Ratón, FL, USA: CRC Press; 2007. p. 130. [ Links ]
Kuchling G. The reproductive biology of the chelonia. Berlin; New York: Springer; 1999. p. 223. [ Links ]
Lombardi J. Comparative vertebrate reproduction. New York, USA: Springer; 1998. p. 469. [ Links ]
Malvasio A, Nascimento-Rocha JMd, Santos HD, Ataídes AGd, Portelinha TCG. Morfometria e histologia das gonadas de machos e femeas recem-eclodidos de Podocnemis expansa e Podocnemis unifilis (Testudines, Podocnemididae). Acta Sci Biol Sci. 2012;34(1):105-112. DOI: 10.4025/actascibiolsci.v34i1.7257. [ Links ]
McArthur S, Wilkinson R, Meyer J. Medicine and surgery of tortoises and turtles. Denmark: Blackwell; 2004. p. 579. [ Links ]
Norris DO, Lopez KH. Hormones and reproduction of vertebrates. Volume 3: Reptiles. Oxford, UK: Academic Press; 2010. p. 406. [ Links ]
Páez VP, Correa JC, Cano AM, Bock BC. A comparison of maternal and temperature effects on sex, size, and growth of hatchlings of the Magdalena river turtle (Podocnemis lewyana) incubated under field and controlled laboratory conditions. Copeia. 2009;4:698-704. DOI: 10.1643/ce08-149. [ Links ]
Páez VP, Restrepo A, Vargas-Ramirez M, Bock BC. Podocnemis lewyana Duméril 1852-Magdalena River Turtle. Chelon Res Monogr. 2009;5:024.1-024.6. [ Links ]
Páez VP, Restrepo A, Vargas-Ramírez M, Bock BC, Gallego-García N. Podocnemis lewyana. In: Páez VP, Morales-Betancourt MA, Lasso CA, Castaño-Mora OV, Bock BC, editors. V. Biología y conservación de las tortugas continentales de Colombia. Bogotá, D. C., Colombia: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH); 2012. p. 528. [ Links ]
Pagliarini Cabral SR, Zieri R, Franco-Belussi L, De Souza Santos LR, Saranz Zago CE, Taboga SR, Oliveira CD. Morphological changes of the epididymis and description of the excurrent ducts of Phrynops geoffroanus (Testudines: Chelidae) during the reproductive cycle. Anat Rec. 2011;294(1):145-155. DOI: 10.1002/ar.21302. [ Links ]
Palmer BD, Guillette LJ. Histology and functional morphology of the female reproductive tract of the tortoise Gopherus polyphemus. Am J Anat. 1988;183(3):200-211. DOI: 10.1002/aja.1001830303. [ Links ]
Pérez ME. Evaluación morfológica del bazo de sapo gigante (Rhinella marina), expuesto a una mezcla de contaminantes en la zona de Coatzacoalcos, Veracruz. Tesis de Maestría en Ciencias Ambientales. San Luis Potosí, México: Universidad Autónoma de San Luis Potosí; 2010. 81 p. [ Links ]
Pérez-Bermúdez E, Ruiz-Urquiola A, Lee-González I, Petric B, Almaguer-Cuenca N, Sanz-Ochotorena A, Espinosa-López G. Ovarian follicular development in the hawksbill turtle (Cheloniidae: Eretmochelys imbricata L.). J Morphol. 2012;273(12):1338-1352. DOI: 10.1002/jmor.20062. [ Links ]
Pieau C, Dorizzi M, Richard-Mercier N, Desvages G. Sexual differentiation of gonads as a function of temperature in the turtle Emys orbicularis: endocrine function, intersexuality and growth. J Exp Zool. 1998;281(5):400-408. [ Links ]
Ravaglia MA, Maggese MC. Melanomacrophage centers in the gonads of the swamp eel, Synbranchus marmoratus bloch (Piscis, Synbranchidae), histological and histochemical characterization. J Fish Dis. 1995;18(2):117-125. DOI: 10.1111/j.1365-2761.1995.tb00269.x. [ Links ]
Romero I. Proyecto de Conservación de la tortuga de río Podocnemis lewyana en la Cuenca baja del río Claro Cocorná Sur. 2011. p. 40. [ Links ]
Sarkar S, Sarkar NK, Maiti BR. Histological and functional changes of oviductal endometrium during seasonal reproductive cycle of the soft-shelled turtle, Lissemys punctata punctata. J Morphol. 1995;224(1):1-14. DOI: 10.1002/jmor.1052240102. [ Links ]
Wyneken J. The anatomy of sea turtles: U.S. Department of Commerce NOAA Technical Memorandum NMFSSEFSC-470; 2001. p. 172. [ Links ]
Yntema CL. Characteristics of gonads and oviducts in hatchlings and young of Chelydra serpentina resulting from three incubation temperatures. J Morphol. 1981;167(3):297-304. DOI: 10.1002/jmor.1051670304. [ Links ]