INTRODUCTION
Due to the rapid depletion of fossil-based fuels in recent years, research has focused on alternative energy sources such as biomass, wind, solar, hydroelectric, geothermal energy and nuclear energy. The study was focused on material aspects such as reducing environmental pollution, appropriate waste management, and sustainable economic development. In this context, it focused on bioethanol, biofuel from biodiesel lipid, waste oil recycling, pyrolysis, gasification, dimethyl ether and biogas. Biofuels are an important potential alternative to fossil fuels in the future [1]. Currently, 88 percent of energy demands are met by burning fossil fuels. CO2 concentrations in our atmosphere have increased from 250 to 400 ppm in a short period of 200 years. In future scenarios, atmospheric CO2 concentration levels are expected to reach approximately 450 ppm by 2100. Global agreements, which involve significant restrictions on anthropogenic Greenhouse Gas (GHG) emissions by the middle of the century, with efforts to keep global temperature change below 2°C before the Industrial Revolution, have been accepted by many countries [2]. Research on sardine fish oil shows that the transesterification method is appropriate in terms of the conversion efficiency of sardine fish oil to biodiesel, reaction time, and compliance of physical and chemical characteristics with ASTM 6751 standard. Fish oil is one of the best sources for alternative fuel [3,4]. Mixing waste sardine fish oil with diesel fuel at 10% improves engine performance, reduces harmful emissions, and such mixtures can also be used without requiring any additional auxiliary systems to the engine [5, 6,]. In the sardine fish oil transesterification method, a maximum ester yield of 98.37% was obtained after a 30-minute reaction period with 1.5% KOH catalyst of fish oil. In the mixtures made with B20, B40, B60, B80 and B100 diesel fuel, there was an increase in BSFC and NOx amounts, and a significant decrease in CO2, HC and smoke emissions as the amount of sardine methyl ester increased [7]. However, in the direct use of raw sardine fish oil in diesel engines, the maximum post-combustion pressure and heat release rate were lower than diesel fuel [8]. Sardine fish oil methyl ester produced by the transesterification method was tested in an engine with variable compression rates. While thermal efficiency was high at low compression rates, BSFC was low, and NOx was low at high compression rates [9]. In some studies, fish oil methyl ester was mixed with diesel fuel at 20% volumetric rate and aluminium oxide and titanium nanoparticles were added to the mixture. The researchers observed significant reduction in NOx and CO2 emissions at full load conditions. It has been found that oil produced from waste fish oil can be used in a diesel engine without any engine changes. Biofuels are alternative fuels that are suitable for use in diesel engines. Fish oil produced from waste fish is a biodiesel renewable energy source. Nanoparticles can also be used as additives in diesel and biodiesel to increase full combustion of fuel and significantly reduce harmful exhaust emissions. [10-12]. It has been observed that the methyl ester produced from Amazon catfish in European Norm (EN-14214) standards complies with the standard in all specifications, except for the content of polyunsaturated methyl esters [13]. 50% canola oil methyl ester was mixed with 50% diesel fuel and 16 ppm organic resin-based manganese was added to this test fuel and tested for engine performance and emissions under full load conditions. Decrease in engine torque and power, CO, HC and % smoke density and increase in CO2 and NOx amounts were detected. Metallic fuel additives improved the characteristics of biodiesel fuels such as pour point and viscosity values [14-15-16]. Methyl esters obtained from waste fish oil and edible oils by transesterification method were placed into diesel fuel at 25% by volume and in engine performance and exhaust emission tests, fish oil biodiesel gave better engine performance and exhaust emission values than fuel oil [17]. Preheating of fish oil biodiesel blends can improve fuel properties. Engine exhaust heat can be used for preheating. In preheating, especially at high temperatures, viscosity and density decrease. [18]. For the synthesis of biodiesel, a two-stage catalytic process was selected, and the chicken oil methyl ester produced in the reaction using methanol, sulfuric acid and sodium hydroxide catalyst in the reaction was mixed with 10% diesel fuel and 12 mol organic-based synthetic manganese was added and examined for engine performance and emissions. In the study, it was observed that the engine torque was not affected by the tests carried out at full load conditions at 1800 and 3000 rpm speeds, the specific fuel consumption increased by 5.2%, smoke and CO emissions decreased, and NOx emissions increased [19]. In biodiesel mixtures, ignition delay and burning time are shorter and this causes lower heat. Injection timing delay affects post-combustion pressure, NOx, HC, CO, emissions, and reduction of combustion parameters [20]. Two-stage processes, esterification and transesterification were implemented to convert waste fish oil into biodiesel. An acid catalyst (H2SO4) and alkaline catalysts were used for esterification and transesterification catalysts, respectively. An efficiency of 66.09% was achieved in biodiesel conversion using methanol from alcohol types [21]. In diesel fuel produced by mixing canola oil methyl ester with fish oil methyl ester at the rates of 10%, 20%, 30%, 40%, 50% and 100% by volume, CBFM (Composite Blends of Fuel Mixtures) fuel was created. This fuel was tested on a single-cylinder engine. As a result of the tests, an increase in brake specific fuel consumption and decrease in CO and HC emissions ranging from 2.8% to 25.1% and 2.2% to 19.9% were determined, respectively [22]. 20% fish oil methyl ester and 20% jatropha oil methyl ester were mixed in diesel fuel and both were tested in a single cylinder engine and compared with diesel fuel in terms of engine performance and emissions. Jatropha oil methyl ester results were better than with fish oil methyl ester. It is to be noted that HC and CO amounts decreased and NOx levels increased in both fuel mixtures compared to diesel fuel. [23]. According to the report published by the Food and Agriculture Organization of the United Nations in 2018, 106,780 tons of sardines were produced in Europe between 2015 and 2016 [24]. According to the data of the Turkish Statistical Institute, sardine fish production was 18,162.1 tons in 2016, 23,425.7 tons in 2017, and 18,854.0 tons in 2018, respectively [25]. The fact that sardine fish wastes have not been evaluated will increase CO2 emissions as well as physical environmental pollution. B25, B50, B75, B100 biodiesel mixtures obtained by mixing fish oil methyl ester with certain proportions of diesel fuel were tested on a single-cylinder diesel engine and BSFC and brake thermal efficiency of B100 fuel was higher than diesel fuel. A higher smoke density, NOx, CO and HC appeared in tests using all fish oil [26]. Considering that 1 kg of raw sardine fish oil waste is obtained from an average of 10 kg of waste from sardine waste (fish heads, fish bones, and fish internal organs), it can be said that sardine fish waste has a high biodiesel potential. The aim of this study is to examine the usability of sardine oil methyl ester doped with manganese-based organic compounds on diesel engines as alternative fuel. Sardine fish oil methyl esters were produced by a double catalysis method, with transesterification, mixed into diesel fuel at different rates, and in the engine tests and emission measurements, it was determined that it was an alternative fuel for reducing harmful emissions compared to diesel fuel.
In this paper, unlike other studies, raw fish oil was obtained from the waste of sardines, not from the fish itself (fish heads, bones and internal organs). Waste sardine oil methyl ester was produced from the raw sardine oil, using the two-stage transesterification method. The produced methyl ester was mixed with 50%, 75% and 100% diesel fuel, and 12 ppm organic resin-based manganese was added to each fuel mixture, and its effects on engine performance and emissions were examined. Therefore, the aim of this study is to determine the biodiesel fuel potential of sardine fish waste (fish heads, bones and internal organs) and to show for the first time that the addition of 12 ppm of manganese on sardine fish waste oil biodiesel has an effect on engine performance and emissions.
2 MATERIALS AND METHODS
In this study, cleaned sardine fish waste (fish heads, bones, internal organs) obtained from Odemis/Izmir fish market were cleaned by pre-washing with cold water and then boiled in a boiler with water that is solvent at a 1:1 rate. After cooling, the fish oil, which was added to the water surface, was collected from the upper part and the filter was made by heating at 30 °C and removing solid materials. 1 kg of raw sardine fish oil was obtained from an average 10 kg of sardine fish waste. With this method, 6.3 kg of raw fish oil was obtained and stored in a dark glass bottle. First, the raw sardines were heated up to 110 °C to eliminate the amount of water in the fish oil and letting it cool. As the raw sardine fish oil contains high fatty acids, the biodiesel conversion process was carried out in two stages. In the first phase, 1% (5g) sulfuric acid (H2SO4) and 40% 98% purity methanol (CH3OH) were mixed for at 40°C for % hour as a catalyst for 500 grams of raw fish oil. 500 grams of raw fish oil was added. It was mixed at 60°C for two hours. After two hours, the temperature was increased to 110°C, letting it cool to fully evaporate its water content. After this process, free fatty acid amounts were measured again by titration method, then starting the second phase. 98% purity methanol was used as a reagent at 20% (95.58 g) of this weight amount. Raw fish oil was mixed for % hour at 40 °C in a glass beaker using sodium hydroxide (NaOH) at 0.5% of its weight (2'38 g). Next, at the end of the first phase, the remaining waste sardine fish oil was carefully added into a mixture of sodium hydroxide and methanol. It was mixed at 60°C for two hours. After that, the temperature was raised to 110°C and placed in the separation funnel and left to rest for 24 hours. Sardine fish oil waste was separated from the methyl ester by removing the glycerine accumulated from the separation funnel tap at the bottom. The separated waste sardine fish oil methyl ester was washed three times with 100°C water. It was then heated to 110°C,the water content evaporated and it was ready for use after cooling. Figure 1 shows the glycerine (dark color, high density) remaining in the lower part of the separation funnel after 24 hours of rest and the waste sardine fish oil methyl ester (light color, low density) obtained in the upper part are separated from each other and the waste sardine fish oil methyl ester is heated to 110°C after washing three times with 100°C distilled water and then left to rest for measurement preparations. To determine the physical and chemical characteristics of the fuels, viscosity, glare, density and freezing tests were carried out in the biodiesel laboratory established within the scope of the project at Manisa Celal Bayar University, Akhisar Vocational School, Manisa-Turkey.
A bomb calorimeter was used to determine the thermal values of the fuels. Manganese dioxide (MnO2) with 98% purity was used as manganese. As an organic structure, the additive was synthesized with a magnetic stirrer under a condenser at about 180°C in the presence of sulfuric acid catalyst in the abietic in the resin in the spindle oil. The solution was prepared with ethanol and doped with methyl ester fuels.
The first experiment fuel (Smn50) was formed by mixing 50% diesel fuel and 50% Sardine methyl ester with sardine fish oil, methyl ester, volumetric diesel fuel (DF) obtained by transesterification method, (Smn75) fuel was obtained by mixing 75% Sardine methyl ester and 25% diesel fuel, (Smn100) fuel mixes were created by mixing 100% Sardine methyl ester and 0% diesel fuel. 12 ppm organic resin-based manganese was added to each fuel mixture. The value of 12 ppm organic resin-based manganese was determined by previous studies at laboratory conditions [15, 27]. It has been found that it lowers the freezing point of organic resin-based manganese, reduces the viscosity and flash point, and decreases the effects of harmful exhaust emissions. It has been determined that organic-based manganese gives the best results in synthesizing organic compounds of Mn, Mg, Cu and Ca metals and using their solutions as additives in diesel fuel [14,28]. Biodiesel fuels obtained after the addition of manganese were tested under full load conditions in a single cylinder diesel engine and compared with pure diesel fuel in terms of engine performance, exhaust emissions, and exhaust temperatures.
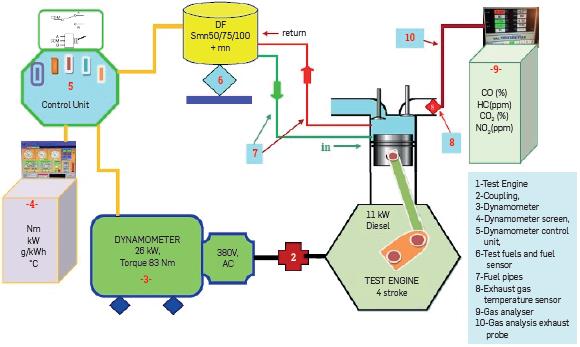
3 EXPERIMENTAL PROCEDURE AND EQUIPMENT
Figure 2 is a flow chart showing the stages of biodiesel production from sardine fish waste. Prior to the experiments, the engine was run until the temperature of the exhaust gas was 300°C to reach the regime temperature and measurements with this reference. In the experiments, after reaching the engine regime temperature, the engine speed was maximized, and a load was applied via the dynamometer, using 3500 rpm as the starting speed for the test. A counter load is applied on the dynamometer until the engine speed drops to 3250 rpm and engine performance (torque-power-specific fuel) values are recorded at every 250 rpm interval. Emission values are recorded at full load in every 500-rpm range.
Technical characteristics of the test engine, engine dynamometer and exhaust gas analyzer are shown in Table 1, Table 2 .
The loading process on the dynamometer was continued until the engine speed reached 1250 rpm. As the engine vibration increased after this speed, the test was terminated at 1250 rpm.
Exhaust emission measurements were done with Bilsa brand MOD 2210 WINXP emission device. Technical characteristics of the exhaust gas analyzer are given in Table 3. The device filter was renewed before every measurement. The exhaust probe and its tube were cleaned after every three measurements. Measurements were recorded as instant data. Measurements comply with TS (Turkish Standards) ISO (International Organization for Standardization) 3930 standard.
4. RESULTS AND ANALYSIS
In this study, sardine fish waste methyl ester obtained from sardine fish waste was mixed with diesel fuel at the rates of 50% (Smn50), 75% (Smn75) and 100% (Smn100) by volume. 12 ppm organic resin-based manganese (Mn) was added to this mixture, and it was examined for engine performance and emissions.
LABORATORY PERFORMANCE TEST RESULTS
The amount of raw fish oil remaining from the 1st stage was determined as 477.89 grams. The obtained free oil rate of the raw sardine fish oil waste was measured by titration method. Free fatty acids increase soap formation and reduce the rate of biodiesel conversion. The amount of free oil acid of biodiesel raw material oil is expected to be less than 2% and, preferably, below 1% [20, 29-31]. For the production of methyl ester, 20% of the crude oil weight was determined as the amount of catalyst to be used in the conversion of 95.58g methanol and 0.5% 2.38g sodium hydroxide (NaOH) methyl ester. Table 4 shows the physical and chemical characteristics of crude sardine fish oil, diesel fuel and fuel mixtures used in this study. In Table 5, the characteristics of the fish oil methyl ester obtained after transesterification are shown and compared with those of other fish oil methyl esters found in the literature.
ENGINE PERFORMANCE TEST RESULTS
Figure 4 shows the engine torque changes. An increase is observed in all fuel mixtures until the maximum torque is reached. Then, a gradual decrease is observed. The torque decreased as the amount of biodiesel increased in fuel mixtures. On average, torque at all engine speeds decreased by 11.64% for Smn50 fuel, 16.71% for Smn75 fuel and 22.63% for Smn100 fuel, compared to DF fuel. At 2000 rpm, the maximum torque was measured as 39.27 Nm for DF fuel, 35.26 Nm for Smn50 fuel, 33.76 Nm for Smn75 fuel and 31.84 Nm for Smn100 fuel. The lowest torque value was obtained in Smn100 fuel. The main reason for the torque and power losses in biodiesel fuels is that the thermal value of sardine fish waste oil biodiesel fuel is low compared to diesel fuel. The low thermal values of biodiesel fuels cause a decrease in torque. Fuel characteristics such as density, kinematic viscosity affect the rate of fuel-air mixture, which affects torque and power. The addition of organic resin-based manganese had no effect on torque and power [16,26,33].
In Figure 5, the power changes of the biodiesel mixtures according to the engine speed compared to the DF fuel can be observed.
Engine power varied depending on the biodiesel mixture rate. As the biodiesel mixture rate increased, engine power decreased. On average, engine power decreased by 11.16% for Smn50 fuel, 16.03% for Smn75 fuel and 19.48% for Smn100 fuel compared to DF fuel. The lowest power value was obtained in Smn100 fuel. At 3000 rpm, maximum power was measured as 10.65 kW for DF fuel, 9.66 kW for Smn50 fuel, 9.22 kW for Smn75 fuel and 8.93 kW for Smn100 fuel. The calorific value of the fuel, fuel atomization, ignition delay, engine load, intake of fresh air, fuel density, and viscosity are the factors that directly affect combustion. This affects torque and power [32,34].
Figure 6 shows the Brake specific fuel consumption (BSFC) changes of the test fuels. At 2000 rpm (the maximum torque speed), was measured as 298.237 g/kW-h in Smn50 fuel, 324.694 g/kW-h in Smn75 fuel, 352.051 g/kW-h in Smn100 fuel and 246.672 g/kW-h in DF fuel. Brake specific fuel consumption at all engine speeds was 20.90% higher on Smn50 fuel, 31.63% higher on Smn75 fuel and 42.70% higher on Smn100 fuel than on DF fuel. Sardine fish oil biodiesel fuel density is higher than DF fuel. Due to the density difference in volumetric mixtures, it also increases the unit mass amount at the same injection pressure. This increases the consumption of specific fuel. Sardine fish oil causes the BSFC value to be high at all speeds and loads compared to DF fuel as a result of the low calorific value of biodiesel fuel compared to DF fuel [13,35]. Similar trends have been demonstrated by other researchers who have studied fish oil biodiesel.
In the study conducted by P. Arul Franco and Narasiman about V sardine fish oil, BSFC values were higher than diesel fuel [9,37,38]. Similar results were obtained in research conducted with other types of fish oil biodiesel. In the engine performance and emission research of biofuels produced from edible and fish oil, BSFC in test fuels was 5.69% higher than diesel fuel [17]. Comparing the mixtures of fish oil biodiesel (F20) and mahua oil biodiesel (M20) to diesel fuel in terms of performance and emissions in a compression ignition engine, it was concluded that both test fuels, which have lower calorific value compared to diesel fuel, differ in fuel consumption according to their heating value differences [38]. While studying engine performance and emission effects of the waste anchovy biodiesel, BSFC was found to increase by 4.96% due to the low calorific value of waste fish oil biodiesel [26]. They tested marine fish oil biodiesel and frying oil biodiesel produced from marine fish waste with diesel fuel in terms of performance and emissions, and BSFC is high given its low thermal value and high density compared to diesel fuel of test fuels [39]. Manganese and nickel additives were added to the tall oil biodiesel, which was found to be high at rates ranging from 3.22% to 6.00% due to the low calorific value of biodiesel fuels in engine tests. They found that metallic additives reduced some fuel consumption [15]. In this paper, as seen in Table 3, BSFC was high due to the low calorific value of Smn50, Smn75 and Smn100 biodiesel fuels compared to DF fuel. Figure 7 shows the exhaust gas temperatures (EGT) of the test fuels. Exhaust gas temperatures have tended to decrease compared to DF fuel depending on the mixing rate at all speeds and loads. The exhaust temperature of the Smn50 biodiesel fuel is closer to the DF fuel. At 2000 rpm, (the maximum torque speed) was measured as 627.66 °C in Smn50 fuel, 622.36 °C in Smn75 fuel, 618.04 °C in Smn100 fuel and 634.33°C in DF fuel. When we look at all engine speeds, the average exhaust temperature was decreased by 1.05% in Smn50 fuel, by 1.98% in Smn75 fuel and by 2.63% in Smn100 fuel in comparison to DF fuel. The abietic flame temperatures of biodiesel fuels are lower than that of diesel fuel. This causes low exhaust gases. The amount of oxygen contained in the fuel affects exhaust gas temperatures [40,41].
EMISSION TEST RESULTS
In this part, the test fuels like Smn50, Smn75 and Smn100 were compared with diesel fuel (DF) for carbon monoxide (CO), hydrocarbon (HC), carbon dioxide (CO2) and nitrogen (NOx). Emission tests were carried out at full speeds in 500 speed intervals. In Figure 8 and Figure 9, changes in carbon monoxide (CO) and hydrocarbon (HC) depending on engine speed are observed for each test fuel.
At 2000 rpm (the maximum torque speed), the amount of CO was measured 4.22% in Smn50 fuel, 4.40% in Smn75 fuel, 4.67% in Smn100 fuel and 3.84% in DF fuel. The average CO amount in all speeds was 17.53% higher in Smn50 fuel, 25.51% higher in Smn75 fuel and 33.23% higher in Smn100 fuel than in DF fuel.
Considering the emission output of HC, at 2000 rpm (the maximum torque speed) was measured as 28.29 ppm in Smn50 fuel, 32.58 ppm in Smn75 fuel, 38.11 ppm in Smn100 fuel and 24.14 ppm in DF fuel. When considering all engine speeds on average, the amount of HC was 13.40% higher in Smn50 fuel, 34.57% higher in Smn75 fuel and 54.87% higher in Smn100 fuel than DF fuel.
The CO and HC values of Smn50, Smn75 and Smn100 fuels were higher in all speeds and loads compared to DF fuel. High CO and HC are chemical energy of unburned fuel. Injection and combustion characteristics of the test engine may be changed depending on the different fuel properties.[17,48]. High viscosity and density of fuel in biodiesel mixtures affect atomization of fuel in cylinders [49]. The higher CO emissions may be due to their greater oxidation as compared to diesel [12]. Smn50 fuel produced less CO and HC than other fuels. CO, unburned hydrocarbons (HC) and NOx and smoke density pollutants originating from diesel engines are the main parameters of the emission standards. CO and unburned hydrocarbon emissions refer to unused chemical energy losses in the engine and an increase in emissions that negatively affect engine performance [20,42,50].
Figure 10 shows the NOx changes of the test fuels compared to the DF fuel. At maximum torque speed, NOx values were measured as 691.89 ppm in Smn50 fuel, 637.99 ppm in Smn75 fuel and 570.61 ppm in Smn100 fuel and 802.08 ppm in DF fuel. In all engine speeds, the NOx value was 10.06% lower in Smn50 fuel, 15.66% ppm in Smn75 fuel and 22.76% lower in Smn100 fuel compared to DF fuel. NOx emissions were lower compared to DF fuel depending on biodiesel mixing rates. The reason may be the excess amount of oxygen that triggers the formation of NOx in the biodiesel. In general, the increase in motor load raises the NOx amount in the right proportion. The increase in engine load increases the overall fuel-air rate, which leads to an increase in the end-of-combustion temperature, and an increase in the amount of NOx [4,13,49]. Cylinder temperature, combustion flame residence time, ignition delay and oxygen concentration are important factors in the formation of NOx concentration. The cetane number affects the NOx level. The amount of NOx decreases in biodiesel fuels with high cetane number [43, 44]. In general, the nitrogen oxide concentration varies linearly with the engine load. As the load increases, the total fuel-air rate increases, causing an increase in the average gas temperature in the combustion chamber and thus NOx, which is sensitive to temperature rise [36]. In this study, the addition of 12 ppm organic resin-based manganese into fish oil methyl ester produced from sardine waste may trigger NOx values positively due to the catalyst effect on the combustion process of metallic additives [45].
In Figure 11, CO2 emission changes of test fuels compared to DF fuel can be observed. CO2 emissions in all biodiesel mixtures were lower than DF fuel. CO2 emissions at maximum torque speed were measured as 10.82% in Smn50 fuel, 10.49% in Smn75 fuel, 10.38% in Smn100 fuel and 10.94% in DF fuel. The average CO2 emission in all engine speeds was 1.25% lower in Smn50 fuel, 3.78% lower in Smn75 fuel and 5.01% lower in Smn100 fuel compared to DF fuel. This is probably due to the low carbon content of biodiesel fuels. The reduction in CO2 concentration was higher at lower engine speeds. The addition of 12 ppm organic manganese triggers the reduction of CO2 emissions. CO2 can be expressed as an indicator of complete combustion [26, 27]. Excessive amount of oxygen in biofuels compared to fossil fuels should improve the combustion [16, 36]. However, as seen in Figure 11, all biodiesel mixtures have lower CO2 emissions than those of DF. This is probably due to the low combustion rates of biodiesel fuels. The amounts of HC and CO in exhaust gases proves these results. Due to the high viscosity and density, oxygen deficiency and semi-oxidation, the reasons for not reaching maximum burning end temperature cause to low CO2 emissions [32, 33].
CONCLUSIONS
In this study, raw fish oil was obtained by using the head, bones and internal organs of the sardine fish collected from the fish market to create alternative fuels for fossil fuels. 1 kg of raw fish oil was produced from an average of 10 kg of sardine fish waste. The free fatty acid amounts of raw fish oil produced were high and the methyl ester of sardine fish waste was produced through a two-stage catalytic process. The obtained methyl ester was mixed with diesel fuel (DF) in certain proportions (50-75% and 100%) in volume, and 12 ppm organic resin-based Manganese (was added into each mixture and Smn50, Smn75 and Smn100 fuels were obtained. The physical and chemical characteristics of the obtained fuels were measured. Experimental fuels were tested on a single-cylinder diesel engine connected to a dynamometer at full load conditions, and engine performance and emission effects were examined. The main findings of this research can be summarized as follows:
 Since the produced raw fish oils have high free fatty acids, two-stage transesterification increases the efficiency of methyl ester.
Since the produced raw fish oils have high free fatty acids, two-stage transesterification increases the efficiency of methyl ester.
 Sardine fish waste methyl ester did not cause any problems in the engine at 100% usage.
Sardine fish waste methyl ester did not cause any problems in the engine at 100% usage.
 The use of Smn50, Smn75 and Smn100 test fuels with 12 ppm organic resin-based manganese compared to DF fuel;
The use of Smn50, Smn75 and Smn100 test fuels with 12 ppm organic resin-based manganese compared to DF fuel;
Decrease in torque, power and exhaust gas temperature values,
Increase in brake specific fuel consumption,
Increase in CO, HC values,
Decrease in NOx and CO2 and emissions observed.
 With all engine speeds, the NOx value was 10.06% lower in Smn50 fuel, 15.66% lower in Smn75 fuel and 22.76% lower in Smn100 fuel compared to DF fuel.
With all engine speeds, the NOx value was 10.06% lower in Smn50 fuel, 15.66% lower in Smn75 fuel and 22.76% lower in Smn100 fuel compared to DF fuel.
 The average CO2 emission at all engine speeds was 1.25% lower in Smn50 fuel, 3.78% lower in Smn75 fuel and 5.01% lower in Smn100 fuel compared to DF fuel.
The average CO2 emission at all engine speeds was 1.25% lower in Smn50 fuel, 3.78% lower in Smn75 fuel and 5.01% lower in Smn100 fuel compared to DF fuel.
 The addition of 12 ppm organic manganese improved NOx and CO2 exhaust emissions.
The addition of 12 ppm organic manganese improved NOx and CO2 exhaust emissions.
 Sardine fish waste has biodiesel potential and can be used as an alternative fuel. Research for other fish waste may expand.
Sardine fish waste has biodiesel potential and can be used as an alternative fuel. Research for other fish waste may expand.