Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
DYNA
Print version ISSN 0012-7353
Dyna rev.fac.nac.minas vol.83 no.198 Medellín Sept. 2016
https://doi.org/10.15446/dyna.v83n198.55559
DOI: http://dx.doi.org/10.15446/dyna.v83n198.55559
The mechanical properties of Portland cement mortars blended with carbon nanotubes and nanosilica: A study by experimental design
Propiedades mecánicas de morteros de cemento Portland adicionados con nanotubos de carbono y nanosilica: Estudio por diseño de experimentos
Oscar A. Mendoza-Reales a, Germán Sierra-Gallego a & Jorge I. Tobón a
a Facultad de Minas, Universidad Nacional de Colombia, Medellín, Colombia. oamendoz@unal.edu.co, geasierraga@unal.edu.co, jitobon@unal.edu.co
Received: February 2nd, 2016. Received in revised form: April 07th, 2016. Accepted: May 11th, 2016.
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract
The pozzolanic effect of nanosilica (NS) particles when combined with Multi Walled Carbon Nanotubes (MWCNT) was studied in Ca(OH)2 pastes and Portland cement mortars. Experimental design techniques were used to plan the experiments and identify the effect of the nanoparticles on the properties of the cementing matrices by means of Analysis of Variance (ANOVA). Samples were prepared with different combinations of NS and MWCNT. Ca(OH)2 pastes were used to study the effect the nanoparticles had on the Calcium-Silicate-Hydrate (C-S-H) production. Portland cement mortars were used to study the effect of the nanoparticles on the compressive and flexural strength of the cementing matrices. We found that only NS had a significant effect on the C-S-H formation for up to 21 days of hydration, and that MWCNT did not present a positive effect on the mechanical strength of mortars due to the effects of reagglomeration.
Keywords: Carbon nanotubes; nanosilica; hybrid effect; pozzolanic activity; strength.
Resumen
El efecto puzolánico de partículas de nanosílice (NS) combinadas con Nanotubos de Carbono de Pared Múltiple (MWCNT) fue estudiado en pastas de Ca(OH)2 y morteros de cemento Pórtland. Técnicas de diseño experimental fueron aplicadas para planear los experimentos e identificar el efecto de las nanopartículas sobre las propiedades de las matrices cementantes usando Análisis de Varianza (ANOVA). Se prepararon muestras con diferentes combinaciones de NS y MWCNT. Las pastas de Ca(OH)2 fueron usadas para estudiar el efecto de las naopartículas en la producción de Silicato de Calcio Hidratado (C-S-H), y los morteros de cemento Pórtland se usaron para estudiar el efecto de las nanopartículas en las resistencias a compresión y flexo-tracción de las matrices. Se encontró que solo la NS tuvo un efecto significativo sobre la producción de C-S-H hasta 21 días de hidratación, y que los MWCNT no presentaron un efecto positivo sobre las propiedades mecánicas de los morteros debido a efectos de reagomeración.
Palabras clave: Nanotubos de carbono; nanosílice; efecto híbrido; actividad puzolánica; resistencia mecánica.
1. Introduction
It is known that the main contribution to the mechanical properties of a hydrated Porltand cement matrix comes from calcium-silicate-hydrate (C-S-H) [1], which is nanometric by nature. Due to the nanometric character of C-S-H, nanoparticles with pozzolanic or reinforcing properties have the most adequate size scale to modify its properties [2]. Additions of nanosilica (NS) are widely recognized for their positive effects on the properties of mortars, especially on its compressive strength [3]. Also, it has been found that the main interaction of carbon nanotubes within a cement matrix is via the C-S-H, which generates a "bridge effect" that improves the tensile load distribution within the matrix and its overall flexural strength [4].
To improve the properties of a cement based matrix using nanoparticles, one of the main issues that has to be addressed is the adequate dispersion of the nanoparticles throughout the matrix [5]. Depending on the type, amount and pH of the media, among other factors, nanoparticles are more prone to form agglomerations [6-7]. When the nanoparticles are not adequately dispersed in the matrix, they act as filler in the nano pores. When properly dispersed, nanoparticles act as nucleation spots for hydration products, accelerating the hydration process, increasing the viscosity of the suspension, helping to maintain the cement grains and aggregates suspended, decreasing the vulnerability to segregation, and increasing the workability of the system [1]. In some cases, it has been reported that poorly dispersed nanoparticles have a tendency to worsen the properties of the cement based matrices [8].
Previous research has been carried out with multi walled carbon nanotubes (MWCNT) dispersed in water using a policarboxilate based superplasticizer (SP) as a dispersing agent. In this research it has been found that alkaline environments, such as those formed during the hydration of cement, which are rich in Ca(OH)2, affect the stability of the MWCNT dispersions and generate agglomeration phenomena due to an interaction between MWCNT and the Ca(OH)2. This interaction prevents electrostatic repulsion between the functional groups on the MWCNT and the SP molecules [9]. Additionally, it has been found that the MWCNT and NS combinations have an accelerating effect on the kinetics of the hydration reaction during the first 24 hours. This has been associated with MWCNT working as nucleation spots for the hydration products generated by the NS. Due to the presence of Ca(OH)2 in the media since the first hours of hydration, the MWCNT dispersed in water will suffer a reagglomeration process. This decreases the specific surface area available for the MWCNT to work as nucleation spots and eventually inhibits the pozzolanic activity of NS. This is reflected in the amount of C-S-H produced and the amount of heat released during the hydration reaction [9-10]. This work studies the effect of NS and MWCNT combinations on the pozzolanic activity of NS and the mechanical properties of Portland cement using factorial experimental designs as a statistical tool to interpret the analysis and the results.
2. Methodology
The materials used in the experimental campaign were multi walled carbon nanotubes (MWCNT) functionalized with acid groups produced by Nanoamor, nanosilica particles (NS) dispersed in water produced by BASF Chemicals, reactive grade Ca(OH)2 produced by Merck, a superplasticizer additive (SP) produced by BASF Chemicals, and type III Portland cement (Cto) produced by Cementos Argos. Aqueous dispersions of MWCNT with a solids concentration of 0.25% wt were produced using an ultrasonic tip processor and SP as a dispersing agent in a 4:1 proportion of SP to MWCNT. The aqueous dispersions were sonicated in 20 second on/off cycles to avoid any overheating until a total amount of energy of 40,000 J was applied to the system. Details of how the dispersions parameters were determined as well as the quantification of the obtained dispersion degree have been published separately [9].
Ca(OH)2 pastes with a water-to-Ca(OH)2 ratio of 1 were produced to identify the influence of MWCNT and NS on the production of C-S-H due to the pozzolanic activity of the NS particles. Due to the fact that both MWCNT and NS were dispersed in water, there is a maximum amount of solid nanoparticles that can be added to the Ca(OH)2 paste without surpassing the fixed water-to-Ca(OH)2 ratio. Taking into account that the solid concentration of the aqueous MWCNT dispersion was 0.25%, and the solid concentration of the NS aqueous dispersion was 49.5%, it was calculated that the maximum possible solid additions of MWCNT and NS were 0.025% and 9.5%, respectively. The pastes were prepared first by mixing the liquid components in a glass beaker and later by adding Ca(OH)2, which was hand-mixed with a spatula until a homogeneous paste was obtained. Finally, the samples were stored in airtight plastic containers that were to be tested after 1, 3, 7, and 21 days of hydration. At each testing age the hydration of the pastes was stopped by grinding them in acetone, oven drying at them at 60 °C, and sieving them though a No 200 mesh. The response variable chosen was the mass loss at 230°C, which was measured in a Precisa Master XM 60 moisture analyzer. Each sample was heated at a rate of 85 °C/min up to the maximum temperature, which was held constant for 3.5 minutes or until the sample reached constant mass. We considered that the sample reached constant mass when in a period of 60 seconds the sample lost 2.0 mg or less.
The experiment was planned using a 23 factorial experimental design with two factors and three levels for each factor. The first factor was the amount of MWCNT, and the levels were 0.00%, 0.025% and 0.225%. The second factor was the amount of NS and the levels were 3.5%, 6.5% and 9.5%. The experimental matrix and the randomization of the experimental runs are presented in Table 1. This design was repeated and analyzed separately for the four different curing ages.
Mortars with a water-to-cementing material ratio of 0.5 were prepared according to the procedure established in the ASTM C109/C109M standard. Immediately after mixing, the flow of each mortar was measured using a flow table, following the procedure established in the ASTM 1437 standard. A necessary amount of SP was added to each mortar to obtain a 110 ± 5% flow. For each experimental run and each testing age (1, 3, 7 and 28 days), three 4.0x4.0x16.0 cm prisms were molded. After 24 hours, the prisms were removed from the molds and cured by submersion in lime saturated water until they reached the testing age. When the testing age was reached, each set of samples was taken out of the curing water and immediately tested for compressive and flexural strength according to the procedure established in the ASTM C348 y C349 standards. The experiments were carried out using a factorial 22 experimental design with two factors (MWCNT and NS), and each factor had two levels (0%NS, 7.3%NS, 0%MWCNT and 0.115%MWCNT). The experimental matrix and the randomization of the experiments are presented in Table 2. This design was repeated and analyzed separately for the four different curing ages.
3. Results and discussion
This section presents the results obtained in the experimental campaigns and is divided into the following sub-sections: materials characterization, pozzolanic activity, superplasticizer demand and mechanical properties.
3.1. Materials characterization
Images were taken of MWCNT using transmission electronic microscopy (TEM) in a model TECNAI 20kV FEI microscope that was operated at 200 kV. The sample was dispersed in ethanol and sonicated in an ultrasonic bath for 30 seconds, then a drop of the sample was placed in a No 300 Formvar cupper mesh. The images obtained are presented in Fig. 1. The structures of MWCNT were confirmed and found to be nanometric in diameter and micrometric in length. MWCNT formed agglomerations and entanglements as a consequence of the Van der Waals forces that cause them to attract and agglomerate [11]. A more detailed characterization of the MWCNT and NS that were used in this research can be found in previous publications [9-10].
The chemical composition of MWCNT, NS, and cement was studied by X-Ray fluorescence (FRX), using portable FRX equipment from Bruker model S1. Tests were performed in powder samples without further preparation. The results obtained are presented in Table 3. The main component of MWCNT is carbon, which is not detected by the FRX equipment. From the elements that were detected, the main constituent was Ni, which was used as a precursor in the production of the nanotubes [9]. As expected, cement was found to be composed mainly of CaO, SiO2, and Al2O3. NS was found to be mainly SiO2.
3.2. Pozzolanic activity
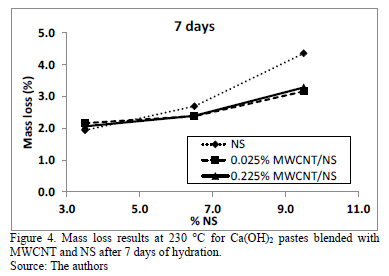
Ca(OH)2 pastes blended with NS and MWCNT in the amounts presented in Table 1 were produced according to the procedure described in the methodology section. After 1, 3, 7, and 21 days of curing, the mass loss of each paste at 230 °C was measured using a moisture analyzer. The results obtained are presented in Figs. 2-5. To validate the use of the moisture analyzer as a method to identify pozzolanic activity, Ca(OH)2 pastes that were blended with different types and different amounts of pozzolans were produced. After the different curing ages the mass loss of each paste was tested at the same temperature by thermogravimetry analysis and by a moisture analyzer. Statistical analysis showed a good correlation between the two testing methods. This validation has been separately published and details can be found in reference [12].
The mass loss results show that for the four ages studied, the higher the amount of NS the higher the mass loss of the pastes. This is caused by the pozzolanic activity of NS, whish is identified as a function of the amount of C-S-H formed by the fixation of Ca(OH)2 by NS [13]. Additionally, for all the NS and MWCNT amounts studied, the mass loss stabilizes at between 2 and 3% from the 7th to the 21st day of hydration. This indicates that in this time interval all the NS reacted to form C-S-H. The addition, MWCNT does not seem to affect the pozzolanic activity of the NS. To confirm this statement, an analysis of variance (ANOVA) was performed, and its results are presented in Table 4.
For the ANOVA, the null hypothesis is that the factors MWCNT and NS do not have any effect over the mass loss of the pastes, and the alternative hypothesis is that the factors do have a significant effect over the mass loss. For a significance of a = 0.05 and an associate confidence b = 95%, it can be said that if a P-value is lower than a, the null hypothesis is rejected. For all the studied ages, the P-value of the factor MWCNT is always higher than 0.05 (accepting the null hypothesis), and the P-Value of the factor NS is lower that 0.005 (rejecting the null hypothesis). This indicates that, with a 95% confidence for the all the tested ages, the addition of MWCNT did not have an effect on the mass loss of the pastes, and all the effects identified are from NS's pozzolanic activity. The experiments presented an average R2 of 88%, which means that the statistical model was capable of accounting for 88% of the variability of the results.
To verify the correct sampling and statistical analysis of the results, the residue plots were verified for the assumptions of normality, constant variance, and independence of the response variable. These plots are not presented here due to space issues. For all the ages, it was concluded that the statistical model was valid because the plot of normal probability was linear, the histogram was Gauss shaped, and the residue versus order and adjustment plots did not show a tendency.
3.3. Super plasticizer demand
To guarantee that the measured strengths of all mortars were comparable, the flow of each mortar was standardized to a 110 ± 5 % value using a superplasticizer (SP), according to the ASTM 1437 standard. The SP demand of each mortar is presented in Table 5. It was found that the samples with NS presented a higher SP demand due to the high specific surface area of the nanoparticles. Mortars with MWCNT did not present a significant SP demand when compared to the plain cement sample because they already contained SP as a dispersing agent. Mortars blended with NS and MWCNT presented a SP demand similar to that of mortars blended only with NS. This indicates that the additive demand of NS is dominant over the presence of SP in MWCNT aqueous dispersion.
3.4. Mechanical strength
The four samples' flexural strength results for the four testing ages are presented in Fig. 6. Each strength result is presented with error bars of one standard deviation. These results were statistically studied using ANOVA. A summary of the P-values obtained for each factor and its interaction are presented in Table 6. For this given ANOVA, the null hypothesis is that the factors MWCNT and NS do not have any effect over the flexural strength of the mortars. The alternative hypothesis is that the factors MWCNT and NS do have a significant effect over the flexural strength of the mortars.
For a significance a = 0.05, i.e. with a confidence b = 95%, it can be said that if the obtained P-value is smaller than a, the hull hypothesis is rejected. This means that the MWCNT did not have any statistically significant effect on the flexural strength at any testing age, while NS only presented an improvement after 1 day of hydration. The MWCNT-NS interaction was statistically significant only after 7 days of hydration. Also, from Fig. 6 it can be seen that its effect was decreasing rather than increasing the flexural strength of the mortar.
The statistical analysis indicates that during the first day of hydration the MWCNT accelerated the pozzolanic activity of NS, which generated an improvement in the flexural strength by working as nucleation points. However, after 3 days of hydration, the improvement disappeared, and after 7 days it there was a decrease in strength. This can be explained by the reagglomeration process of the MWCNT [9-10], which inhibited the activity of the NS by decreasing its available surface area to react. After 7 and 28 days, not only the pozzolanic activity of NS was completely inhibited, but also the hydration process of cement was affected. This decreased the overall strength of the matrix.
Compressive strength results for the 4 samples at the 4 testing ages are presented in Fig. 7. As with the flexural strength, all results are presented with error bars of one standard deviation. The statistical analysis was carried out with the same null and alternative hypotheses used for the flexural strength results. A summary of the P-values obtained for each factor and their interaction are presented in Table 7.
The addition of MWCNT did not have any statistically significant effect on the compressive strength of the samples at any testing age while NS presented a significant improvement only after 1 and 3 days of hydration. Again, this behavior can be explained by the reagglomeration process of the MWCNT. The pozzolanic activity of NS was probably inhibited by the MWCNT agglomeration after 7 and 28 days of hydration.
4. General discussion
From the results, it was verified that only NS presented a significant Ca(OH)2 fixation that translated into a higher C-S-H production; MWCNT had no effect. This confirms the fact that MWCNT have no chemical affinity with the hydration products of cement [14], and any mechanical improvement caused by the MWCNT is a consequence of physical effects such as nucleation or load distribution through a "bridge effect" [4].
Only during early ages were flexural and compressive strengths of the mortars improved by the combination of nanoparticles. At later ages the presence of the combination of nanoparticles was even found to be detrimental. These results are in agreement with previous reports that found that a predominance of a reaglomeration process of MWCNT inhibited the pozzolanic activity of NS and hindered the hydration reaction of cement at ages greater than 24 hours of curing [9-10]. This reagglomeration can account for the decrease in mechanical properties at later ages. The expected positive effects of the MWCNT on the mechanical properties of the cement matrix may have occurred before 1 day of hydration and were not detected in the experiments carried out due to a rapid reagglomeration of the MWCNT.
5. Conclusions
- NS particles maintained their pozzolanic activity up to the 7th day of hydration in Ca(OH)2 pastes while MWCNT did not have any effect on it at any age.
- Mortars with MWCNT did not present a SP demand higher than that of plain cement ones. This is consequence of the presence of SP in the MWCNT as a dispersing agent.
- The addition of MWCNT did not have any significant effect on the compressive strength at any curing age while NS presented a significant improvement after 1 and 3 days of hydration.
- MWCNT did not present positive effects on the mechanical strength of mortars. After 3 days of hydration, they inhibited the pozzolanic activity of NS due to their reagglomeration process, which translated into a decrease in mechanical strength.
References
[1] Tobón, J.I., Payá, J.J., Borrachero, M.V. and Restrepo, O.J., Mineralogical evolution of Portland cement blended with silica nanoparticles and its effect on mechanical strength. Construction and Building Materials, 36, pp. 736-742, 2012. DOI: 10.1016/j.conbuildmat.2012.06.043 [ Links ]
[2] Sanchez, F. and Sobolev, K., Nanotechnology in concrete - A review. Construction and Building Materials, 24(11), pp. 2060-2071, 2010. DOI: 10.1016/j.conbuildmat.2010.03.014 [ Links ]
[3] Hewlett, P., Lea's Chemistry of Cement and Concrete, 4th edition, Oxford: Elsevier Science & Technology Books, 2004. [ Links ]
[4] Li, G., Wang, P. and Zhao. X., Mechanical behavior and microstructure of cement composites incorporating surface-treated multi-walled carbon nanotubes. Carbon, 43(6), pp. 1239-1245, 2005. DOI: 10.1016/j.carbon.2004.12.017 [ Links ]
[5] Yazdanbakhsh, A., Grasley, Z., Tyson, B. and Abu Al-Rub. R.K., Dispersion quantification of inclusions in composites. Composites Part A: Applied Science and Manufacturing, 42(1), pp. 75-83, 2011. DOI: 10.1016/j.compositesa.2010.10.005 [ Links ]
[6] Shih, J., Chang, T. and Hsiao, T., Effect of nanosilica on characterization of Portland cement composite. Materials Science and Engineering: A, 424, pp. 266-274, 2006. DOI: 10.1016/j.msea.2006.03.010 [ Links ]
[7] Srinivasan, S., Barbhuiya, S., Charan, D. and Pandey, S.P., Characterising cement-superplasticiser interaction using zeta potential measurements. Construction and Building Materials, 24(12), pp. 2517-2521, 2010. DOI: 10.1016/j.conbuildmat.2010.06.005 [ Links ]
[8] Morsy, M.S., Alsayed, S.H. and Aqel, M., Hybrid effect of carbon nanotube and nano-clay on physico-mechanical properties of cement mortar. Construction and Building Materials, 25(1), pp. 145-149, 2011. DOI: 10.1016/j.conbuildmat.2010.06.046 [ Links ]
[9] Mendoza, O., Sierra, G. and Tobón, J.I., Influence of super plasticizer and Ca(OH)2 on the stability of functionalized multi-walled carbon nanotubes dispersions for cement composites applications. Construction and Building Materials, 47, pp. 771-778, 2013. DOI: 10.1016/j.conbuildmat.2013.05.100 [ Links ]
[10] Mendoza, O., Sierra, G. and Tobón, J.I., Effect of the reagglomeration process of multi-walled carbon nanotubes dispersions on the early activity of nanosilica in cement composites. Construction and Building Materials, 54, pp. 550-557, 2014. DOI: 10.1016/j.conbuildmat.2013.12.084 [ Links ]
[11] Rausch, J., Zhuang, RC. and Mäder, E., Surfactant assisted dispersion of functionalized multi-walled carbon nanotubes in aqueous media. Composites Part A: Applied Science and Manufacturing, 41(9), pp, 1038-1046, 2010. DOI: 10.1016/j.compositesa.2010.03.007 [ Links ]
[12] Mendoza, O. and Tobón, J.I., An alternative thermal method for identification of pozzolanic activity in Ca(OH)2/pozzolan pastes. Journal of Thermal Analysis and Calorimetry, 114, pp 589-596, 2013. DOI: 10.1007/s10973-013-2973-y [ Links ]
[13] Frias, M., Villar-Cociña, E., Saánchez, M.I. and Valencia-Morales. E., The effect that different pozzolanic activity methods has on the kinetic constants of the pozzolanic reaction in sugar cane straw-clay ash/lime systems: Application of a kinetic-diffusive model. Cement and Concrete Research, 35(11), pp. 2137-2142, 2005. DOI: 10.1016/j.cemconres.2005.07.005 [ Links ]
[14] Makar, J.M. and Chan, G.W., Growth of cement hydration products on single-walled carbon nanotubes. Journal of the American Ceramic Society, 92(6), pp. 1303-1310, 2009. DOI: 10.1111/j.1551-2916.2009.03055.x [ Links ]
O.A. Mendoza-Reales, received a BSc. in Civil Engineering in 2009 and an MSc in Materials Engineering in 2013, both from the Universidad Nacional de Colombia. Medellin, Colombia. Currently he is a doctoral candidate in the Civil Engineering program at the Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil. His research interests include: chemistry of cement, cement hydration, nanotechnology, pozzolanic materials, carbon nanotubes, oil well cementing pastes and environmental impact of cement based materials. ORCID: 0000-0002-4241-1321
G. Sierra-Gallego, received a BSc. in Chemical Engineering in 1991, an MSc in Chemical Sciences in 2003 both from the Universidad de Antioquia, Colombia and a PhD in 2007 from Poitiers University. Currently, he is a Professor in the Materials and Minerals Department, Facultad de Minas, Universidad Nacional de Colombia. His research interests include: materials sciences, synthesis of carbon nanotubes, heterogeneous catalytic processes, biofuels and energetic evaluation of byproducts. ORCID: 0000-0001-7981-2041
J.I. Tobón, received a BSc. in Geological Engineering in 1992, an MSc in Engineering in 2003, and a PhD in Science and Technology of Materials in 2011, all from the Universidad Nacional de Colombia, Medellin, Colombia. From 1992 to 1995, he worked for different companies in mining and oil; from 1995 to 1999 he worked for Cementos Argos S.A., at the same time from 1993 to 1999 he worked for the Universidad Nacional de Colombia as a part-time Professor, and since 1999 he has worked for the Universidad Nacional de Colombia on a full-time basis. Currently, he is a full professor in the Materials and Minerals Department, Facultad de Minas, Universidad Nacional de Colombia. His research interests include: industrial application of minerals and rocks, chemistry and mineralogy of cements, nanotechnology in construction materials, alternative cementitious materials, and high performance cements and concretes. ORCID: 0000-0002-1451-1309