Introduction
SARS-CoV-2 is a non-segmented, enveloped RNA virus belonging to the Coronaviridae family of the order Nidovirales. This virus has high similarity to other coronaviruses reported in the past (20-60% with MERS-CoV and 45-90% with SARS-CoV) and is responsible for the 2019 coronavirus disease (COVID-19).1-6SARS-CoV-2 uses the angiotensin-con-verting enzyme 2 (ACE2) receptor as the main entry into cells; this receptor is found in the mucosa of the respiratory tract, vascular endothelial cells, heart, intestine, and kidney.7,8 The clinical manifestations of COVID-19 vary considerably, ranging from asymptomatic patients to cases presenting with multiple organ dysfunction leading to death.8-11
SARS-CoV-2 infection in the perinatal period has raised concerns about potential outcomes for both pregnant women and newborns (NB)12,13 because the changes that occur over the course of COVID-19 in the cardiorespiratory and immune systems increase the susceptibility of pregnant women to respiratory infections. In addition, due to the effect of estrogens in the nasopharynx, one fifth of pregnant women develop gestational rhinitis, which can mask the coryza of this disease.14,15According to Narang et al.,13 the incidence of preeclampsia also increases in pregnant women infected with SARS-CoV-2 due to the interaction of the virus with ACE2. Moreover, as reported by Juan et al.,16 this virus can cross the wall of the chorionic villi and cause vertical transmission.
Consequently, the objective of the present study was to describe the clinical characteristics and laboratory and imaging findings in pregnant women with COVID-19 and their NB.
Materials and methods
An overview of systematic reviews (SR) was performed. The study protocol is available in the Prospective International Registry of Systematic Reviews (PROSPERO) under code CRD42020178329.17
Search strategy
The acronym POT was used for the preparation of this SR overview, and the letters stand for: P (population): pregnant patients and their NBs; O (outcome): clinical, radiological and laboratory characteristics of pregnant women with a confirmed diagnosis of COVID-19 and their NBs; and T (type of study): SR of observational studies.
The search for SRs was carried out in PubMed, Scopus, Web of Science and Cochrane Database of Systematic Reviews using the following search strategy: publication period: February 1, 2020 to May 30, 2021; types of study: systematic reviews describing clinical features and imaging and laboratory findings in pregnant women diagnosed with COVID-19 and their NBs; language: unrestricted; search terms: "COVID-19", "coronavirus", "coronavirus covid-19", "SARS CoV 2" "pregnant" "pregnant women", "newborn" and "neonate", which were combined using the "OR" and "AND" connectors to establish the search equations. The following validated filters were also used for the search: (((systematic review[ti] OR systematic literature review[ti] OR systematic quantitative review[ti] OR systematic meta-review[ti] OR systematic critical review[ti] OR systematic mixed studies review[ti] OR systematic mapping review[ti] OR systematic cochrane review[ti] OR systematic search and review[ti]) NOT comment[pt] NOT (protocol[ti] OR protocols [ti])) NOT MEDLINE [subset]) OR (Cochrane Database Syst Rev[ta] AND review[pt]) OR systematic review[pt].
Inclusion criteria and quality of evidence
SRs that included primary cohort or case-control studies describing the clinical characteristics and the results of laboratory tests and chest imaging studies (of pregnant women and their NBs) were selected. Moreover, in order to improve the quality of the evidence presented, only those SRs that obtained a score ≥70% in the critical evaluation were included. The validated tool published by the Joanna Briggs Institute18 was used to assess the methodological quality of a study and determine the extent to which a study addressed the possibility of bias in its design, implementation, and analysis.
Similarly, following the recommendations of the Cochrane Manual,19 the quality of the evidence was evaluated for each of the outcomes presented in the SRs included, considering the risk of bias, indirect evidence, inconsistency, and imprecision, in accordance with the indications of the GRADE handbook.20
Search procedure
The present SR overview was carried out as follows: AEHT and CVAJ conducted the database searches, and SVM and PCTP independently selected the articles based on the title and abstract reading and then applied the inclusion criteria to select articles for full-text reading. In case of disagreement, all authors reached a consensus decision. Data extraction and transfer to the spreadsheet base form was performed by CSVT and MJPP. Tables presenting the results were prepared by SVM and CSVT, while the meta-analyses were performed by SVM. All authors participated in the final drafting of the document.
Statistical analysis
The odds ratio (OR) with its 95% confidence interval (95%CI), if available, was obtained for each variable; the number of cases (n) and the total number of observations (N) were also extracted, both to calculate the percentages and to reanalyze the data and confirm the associations.
Bayesian random-effects meta-analyses were performed; they have multiple advantages over the frequentist model, since the graphs accurately explain each parameter and the number of patients, or sample size, does not influence the results. Therefore, this statistical model is considered robust to outliers or heterogeneous values.21-23
In each meta-analysis, Markov chain Monte Carlo methods were applied, which simulate values from the prior distribution and generate a representative sample of values from the posterior distribution; to achieve this, four chains were structured, and 10 000 interactions were calculated.21,24 The effect size (ES) with its 95%CI quantifies the difference between the groups analyzed. In addition, the forest plots show the values of the estimated ES for each study; the overall ES estimated by the model is represented in the graphs with the Greek letter mu (p), and the OR corresponds to the antilogarithm of p. The Bayes factor for effect size is presented using a sequential plot that shows how the entry of each study determines the direction that the posterior probabilities of the model take, either toward the alternative hypothesis (H1) or toward the null hypothesis (H0).
The Mendeley and Zotero reference managers were used for the management and detection of duplicate articles, and the Rayyan QCRI collaborative web application was used for the selection of SRs.25 Meta-analyses were performed with the statistical software R v4.0.126 and JASP.27
Results
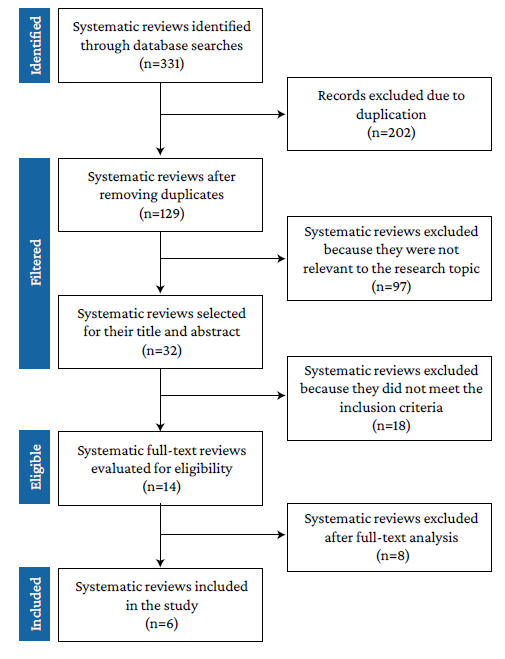
The initial search yielded 331 results, of which 202 were eliminated because they were duplicates, 97 because they did not address the research topic, 18 because they did not meet the inclusion criteria, and 8 because they were not considered relevant to the research objective, so 6 SRs were finally included (Figure 1).
The SRs included described a total of 617 primary articles, of which 14 (0.02%) overlapped. Table 1 presents the main characteristics of the six SRs included in the study.
Table 1 Characteristics of the systematic reviews included in the overview.

* The study by Jafari et al.28 does not report the origin of 5 of the included studies
† In the original study, these data are only reported for 324 of the 349 studies.
‡ Of the 37 articles included, 33 (case reports, national reports, and case series) were used for descriptive analysis and 4 (case-control studies) for meta-analysis.
Source: Own elaboration.
Regarding symptoms in pregnant women with COVID-19, it was found that the percentage of asymptomatic patients ranged from 28.48% to 85.97%; that cough was present in 4L26-48.54%;14,30,31fever, in 17.52-74.98%;14,15,28,31 myalgia, in 18.99-26.51%,14,28and dyspnea, in 5.64%-21.98%.14,28,31In addition, Allotey et al.14 found that 35% (95%CI: 26-45) of patients developed pneumonia and 22% of those patients (95%CI: 12-36) required oxygen; these values were obtained from a single ratio meta-analysis.
The percentage of pregnant women with severe disease or who required admission to the intensive care unit (ICU) ranged from 1.88% to 10.36%.14,30,31Huntley et al.30 reported that maternal sepsis occurred in 3.12% of cases, while Allotey et al.14 reported a much lower incidence of hepatic, cardiac or renal failure in patients, with a range of 0.00-0.13%. The maternal mortality reported by Jafari et al.28 (11.31%) was quite high compared to that reported by Novoa et al.,3 who reported 0.31%, and by Allotey et al.,14 who found a range of0.00-0.08% for this outcome.
The most common comorbidities found were: obesity, diabetes (any type), preeclampsia, hypertensive disorders and asthma, which ranged from 23.17-24.21%,30,313.31-9.98%,28,30,312.34%-9.48%,28,31 2.34%-9.01%,28,30,31 and 7.81%,31 respectively. Other interesting findings were reported by Huntley et al.,30 who found that 33.05% of pregnant women with COVID-19 were older than 35 years and that 8.49% smoked, and by Jafari et al.,28 who reported that 17.05% of pregnant women with COVID-19 were health care workers.
In the SRs reviewed, laboratory tests of pregnant women with COVID-19 reported lymphopenia, leukocytosis, thrombocytopenia and elevated C-reactive protein (CRP) levels in 35.95-64.03%, 9.77-27.41%, 5.68-17.02%, and 46.78-52.27% of cases, respectively.14,28,31 Only Allotey et al14 reported elevated procaldtonin levels in 21% (95%CI: 0-59%) of the pregnant women; in addition, these same authors found abnormal liver function tests in 13% (95%CI: 6-21%). In turn, Novoa et al.31 found elevated transaminase levels in up to 9% of pregnant women. Abnormalities in chest x-rays or CT scans were observed in 69%, 89% and 93.91% of pregnant women according to Allotey et al.,14 Jafari et al.,28 and Novoa et al,31 respectively.
Jafari et al.28 reported that 7.99% of NBs were positive for SARS-CoV-2 and that the virus was isolated from placenta, vaginal secretion, umbilical cord, amniotic fluid and breast milk samples in 12.08%, 4.74%, 5.86%, 5.57%, and 4.94%, respectively. Furthermore, according to these same authors, 5.32% of NBs were infected by vertical transmission, there was bacterial coinfection in 16.01% of pregnant women, and viral coinfection occurred in 14.01% of cases, although the germs were not identified.28
Regarding obstetric outcomes, 30.81-54% of the newborns were delivered by cesarean section and 13.31-20.98% were preterm; also, fetal well-being involvement (FWI) occurred in 4.91-15.97% of the cases.14,28,30,31 The 54.51% figure reported by Jafari et al.28 for postpartum bleeding was significantly higher than that described in the reviews by Allotey et al.,14 who found a percentage of 8% (95%CI: 3-14), and Huntley et al.,30 who reported that antepartum or postpartum bleeding occurred in 3.96% of cases. On the other hand, Allotey et al.14 and Jafari et al.28 found that the percentage of pregnant women with premature rupture of membranes (PROM) was 5.84% and 14.03%, respectively; in addition, the latter study found that 4.02% of the pregnant women suffered miscarriages.
Regarding neonatal outcomes, Jafari et al.28 reported that 55.84% of infants born to mothers with COVID-19 received breast milk substitutes, while 38.96% received mixed feeding. Admission to neonatology units was reported in 12.75-33% of cases.14,28,30Jafari et al.28 also stated that the percentage of stillbirths and neonatal mortality was 4.04% and 2.51%, respectively, the latter figure being considerably higher than that reported by Allotey et al.14 and Novoa et al.,30 who reported <1%. Huntley et al.30 reported that 11.57% of the NBs were small for gestational age, while those large for gestational age accounted for 8.94%. Furthermore, in that study, NBs requiring mechanical ventilation >6 hours accounted for 6.06%.
Table 2 presents an overview of the SRs comparing pregnant and non-pregnant women with COVID-19,14,15,28,31while Table 3 presents an overview of the SRs comparing pregnant women with and without COVID-19.14,28-31
Table 2 Comparison: pregnant and non-pregnant women with COVID-19.
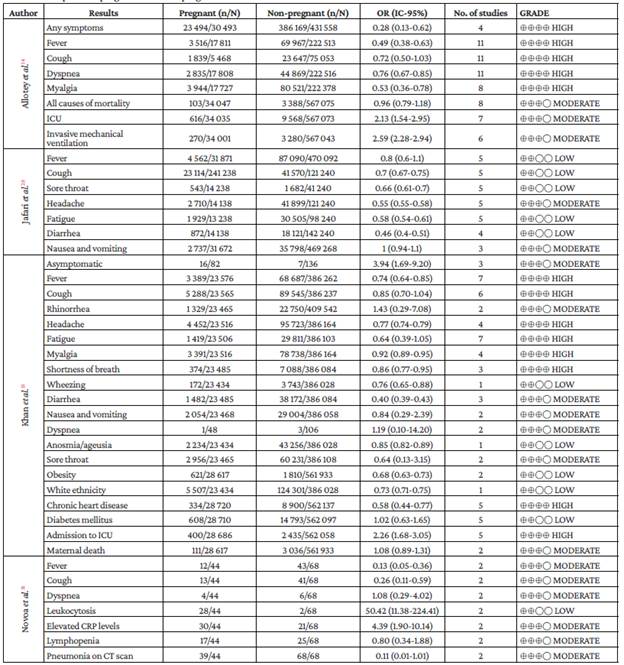
n: number of cases; N: total number of observations; OR: Odds ratio; No. of studies: number of primary studies describing this variable; ICU: intensive care unit; CRP: C-reactive protein; CT: computed tomography.
Source: Own elaboration.
Table 3 Comparison: Pregnant women with and without COVID-19.
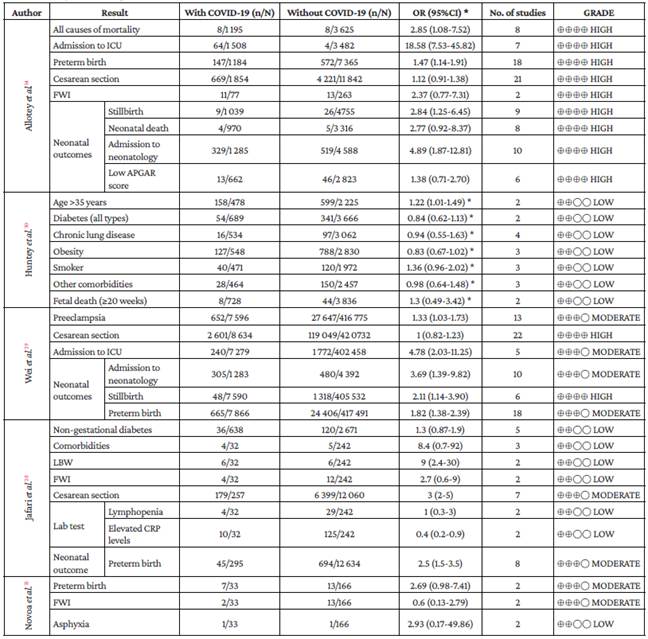
n: number of cases; N: total number of observations; OR: Odds ratio; No. of studies: number of primary studies describing this variable; ICU: intensive care unit; LBW: low birth weight; FWI: fetal well-being involvement.
* Regarding the study by Huntey et al.,30 the OR values (95% CI) were calculated by the authors of the present study based on the data available in the cited paper.
Source: Own elaboration.
Allotey et al.14 studied the association between some risk factors or comorbidities and the possibility of presenting severe COVID-19 disease, admission to the ICU, invasive mechanical ventilation (IMV) or maternal death; the details of these results are presented in Table 4.
On the other hand, Wei et al.29 analyzed the results of systematic reviews comparing outcomes in pregnant women with severe and mild COVID-19 disease. (Table 5)
Table 4 Comparison: pregnant women with severe COVID-19 disease (intensive care unit admission, invasive mechanical ventilation or maternal death) according to the risk factor described by Allotey et al.14
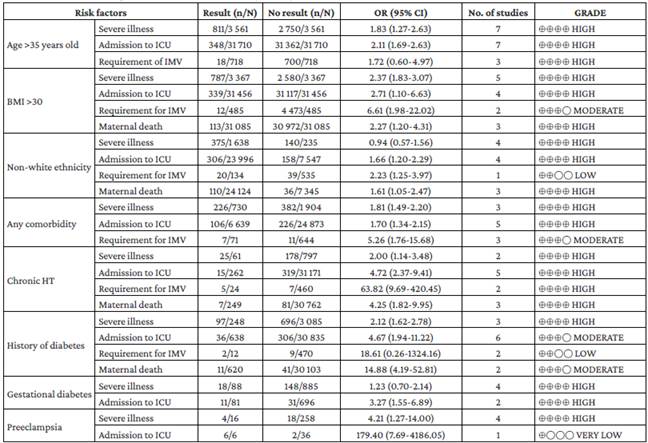
n: number of cases; N: total number of observations; OR: Odds ratio; No. Studies: number of primary studies describing this variable; BMI: body mass index; HT: hypertension; ICU: intensive care unit; IMV: invasive mechanical ventilation.
Source: Own elaboration.
Table 5 Comparison: Pregnant women with severe and mild COVID-19 according to Wei et al.29
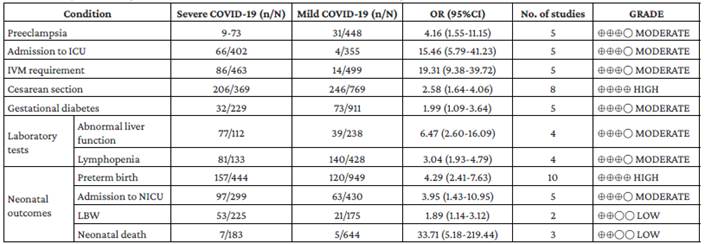
n: number of cases; N: total number of observations; OR: Odds ratio; No. of studies: number of primary studies describing this variable; ICU: intensive care unit; IMV: invasive mechanical ventilation; NICU: neonatal intensive care unit; LBW: low birth weight.
Source: Own elaboration.
Similarly, Wei et al.29 compared groups of pregnant women with symptomatic and asymptomatic COVID-19, as evidenced in Table 6.
Table 6 Comparison: Pregnant women with symptomatic and asymptomatic COVID-19 according to Wei et al.29
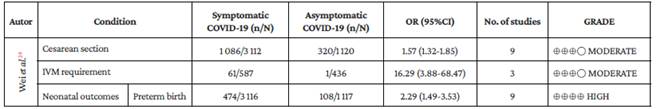
n: number of cases; N: total number of observations; OR: Odds ratio; No. of studies: number of primary studies describing this variable; IMV: invasive mechanical ventilation.
Source: Own elaboration.
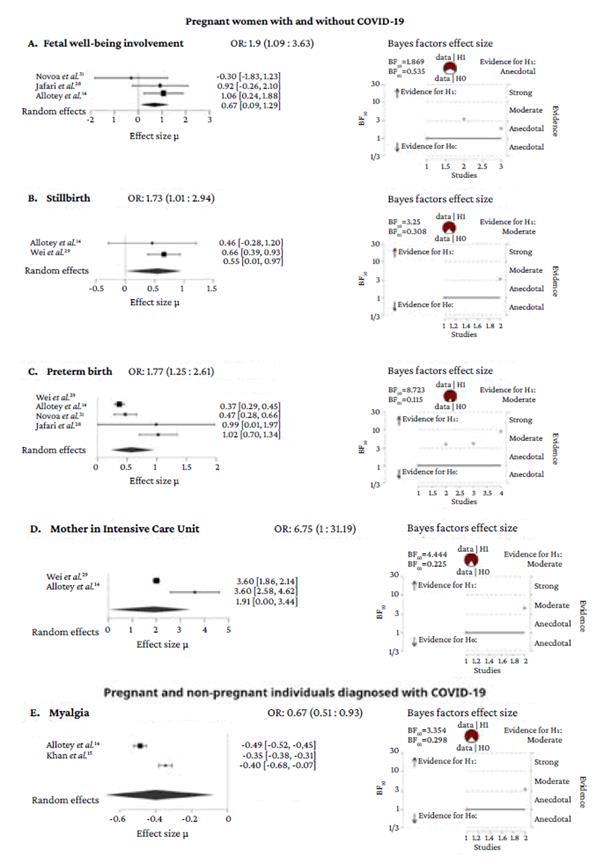
All variables were reanalyzed by means of a Bayesian meta-analysis; however, some of the associations described in the SRs could not be confirmed, although significant heterogeneity was found. Figure 2 includes only the variables that showed associations. It presents, together with the forest plot, the Bayes factor for the effect size. Thus, each graph shows how the evidence in favor for the alternative hypothesis accumulates as the studies are entered. In Figure 2A, the Bayes factor for effect size is anecdotal.
Discussion
The signs and symptoms described in the SRs reviewed in the present study for pregnant women with COVID-19 are similar to those reported in other population groups;32-34however, it was not possible to calculate a general proportion based on these studies due to their high heterogeneity. For this reason, a narrative synthesis of the proportions of clinical characteristics and laboratory and imaging findings in pregnant women with COVID-19 and their NBs was performed.
When the outcomes across the groups studied in each SR were analyzed, it was found that most of the quality of the evidence appears to be moderate or high (Tables 2 to 6). However, the results that could be generalized to the population of pregnant women are those obtained through Bayesian meta-analyses (Figure 2): stillbirth: OR=1.73 (95%CI: 1.01-2.94); preterm birth: OR=1.77 (95%CI: 1.25-2.61); maternal admission to the ICU: OR=6.75 (95%CI: 1-31.19) and fetal welfare involvement: OR=1.9 (IC95%: 1.09-3.63) for pregnant women with and without COVID-19 (although the evidence in favor of H1 is anecdotal for the latter); and myalgia: OR=0.67 (95%CI: 0.51-0.93) for pregnant and non-pregnant women with COVID-19.
Allotey et al.14 and Khan et al.15 found that pregnant women with COVID -19 show a lower proportion of symptoms such as fever, cough dyspnea, myalgia, headache, fatigue and diarrhea compared to non-pregnant women with COVID-19 (Table 2), although the Bayesian meta-analysis could only corroborate this result for myalgia. Moreover, it has been established that respiratory infections caused by SARS-CoV, MERS-CoV and SARS-CoV-2 coronaviruses can be associated with gastrointestinal symptoms such as nausea, vomiting or diarrhea,35-37and that if transaminase levels are elevated, the certainty that SARS-CoV-2 is the causative agent increases.38-41
In order to assess a pregnant woman, the physiological changes typical of this period should be considered, including increased heart rate, increased oxygen consumption, elevation of the diaphragm due to the enlargement of the uterus (which causes some degree of respiratory distress) and relaxin effects (which can cause musculoskeletal pain, especially in the pelvic girdle),42-43as these manifestations may not be perceived as abnormal by pregnant women with mild SARS-CoV-2 infections. At this point, it should be noted that universal screening methods for COVID-19 in high-income countries favored pregnant women, thus detecting a significant number of asymptomatic pregnant women.14,28,44,45
In the present study, leukopenia, lymphopenia, lymphocytosis and elevated CRP levels were found to be the most frequent laboratory alterations in pregnant women infected with SARS-CoV-2. This is consistent with the findings reported by Wang et al.46 in a retrospective study of 72 women with COVID-19 (30 pregnant and 42 non-pregnant) admitted to the Wuhan Central Hospital between December 8, 2019, and April 1, 2020, and by Dubey et al.47 in a systematic review and meta-analysis including 61 studies with a total of 790 women with COVID-19 and 548 NBs. In turn, Papapanou et al.45 in a SR that included 39 studies, reported that D-dimer test results were elevated in 22.30-84.60% of pregnant women; this wide range could be explained by the heterogeneity of the sample size as indicated by the authors in the abstract of their article.
On the other hand, Wei et al.29 found that the likelihood of lymphopenia was three times higher in pregnant women with severe COVID-19 disease (Table 5). Also, Xie et al.,48 in a systematic review and meta-analysis including 90 studies with a total of 16 526 patients with COVID-19, found that 46.50% of cases had lymphopenia, as well as an OR=2.78 (95%CI: 1.85-4.19) for lymphopenia in critically ill adults. Zare et al.,49 in a systematic review that included 660 studies, describe an OR=4.85 (95%CI: 3.31-7.08) for the likelihood of lymphopenia in critically ill adult patients and a very similar value in patients who did not survive COVID-19: OR=4.15 (95%CI: 2.660-6.46). Based on the preceding findings, it is clear that lymphopenia is associated with severe COVID-19 disease. However, increased leukocyte and neutrophil counts, lymphopenia, thrombocytopenia, and increased CRP levels throughout pregnancy and labor are hematologic changes unique to pregnant women that could interfere with the interpretation of laboratory tests during COVID-19.50-52Regarding hematologic manifestations in pregnant women with COVID-19, Servante et al.,53 in a systematic review and critical analysis that included 69 articles, found that the risk of bleeding was higher in this population due to coagulopathies and thromboembolism.
In the present study it was also found that alterations in pregnant women with COVID-19 detected in both x-ray and CT scans of the thorax can occur in up to 93% of cases. However, it has not yet been established whether there is a typical radiological pattern in pregnant women since, so far, the presence of pulmonary consolidation with or without pleural effusion54,55or ground-glass opacity has been described.12,45,56,57
The clinical conditions in pregnant women that were found to be associated with COVID-19 in the present study were maternal age >35 years, overweight, obesity, diabetes, and hypertensive disorders, both chronic and those inherent to pregnancy.14,58-60 Consequently, the presence of these clinical conditions increases the risk of acute respiratory distress syndrome, even in pregnant women with mild COVID-19.61-63Thus, pregnant women with SARS-CoV-2 who present any of the conditions described above are more likely to be admitted to the ICU and require IMV.12,28,34,64
Concerning maternal mortality, different percentages were found in the present study; for example, according to Allotey et al.14 and Jafari et al.,28 it is 0.01% and 11.31%, respectively, although most studies consider that this outcome does not exceed 2%.30,45,58It is noteworthy that this 2% is a relatively low percentage compared to maternal mortality caused by the MERS-CoV and SARS-CoV outbreaks, which was 35% and 10%, respectively.65-67
According to Jafari et al.,28 7.99% of the NBs were positive for SARS-CoV-2, which is notably higher than the rates reported by Woodworth et al.68 and Rodrigues et al.,69 who reported SARS-CoV-2 positivity in 2.60% and 3% of NBs, respectively. In addition, it is important to mention that other SR report an even lower rate of vertical transmission of SARS-CoV-2.45,70-73
Complications such as spontaneous abortions, intrauterine growth restriction and premature delivery have also been attributed to COVID-19;8,64,74however, at the time of this writing, no teratogenic effects produced by SAR-CoV-2 have been identified. Indeed, studies on viral and congenital infections, such as Coyne et al.,75 have established that even in the presence of infections in the placenta or the fetus, the occurrence of a teratogenic process cannot be assured.
It was also found in the present study that up to one third of NBs were admitted to neonatology units, but the same SRs indicate that some admissions were made as a precaution or because of the mother's positive status and not because of the severity of the disease itself, since it has been reported that the occurrence of severe disease due to COVID-19 in NBs is rare.76-79This study also found that up to 54% of newborns were delivered by cesarean section, and that the likelihood of requiring this procedure increased twofold if the mother was symptomatic or had severe COVID-19 disease. In this regard, several authors agree that the sole fact of being positive for SARS-CoV-2 is not an indication for cesarean section, since this procedure increases the risk of morbidity in both the mother and the nb.31,45,57,77,80,81
Before COVID-19 was declared a pandemic, the percentage of preterm births in Latin America ranged from 5% to 18%.82 In the present study, the percentage of preterm births in pregnant women with COVID-19 was found to be as high as 20.98%, with an OR=1.77 (95%CI: 1.25-2.61); however, it remains to be defined whether this increase in preterm births is a consequence of the virus or is related to the high number of cesarean sections performed.72
The worldwide prevalence of perinatal asphyxia is estimated at around 20 cases per 1 000 live births (2%);83 in pregnant women with COVID-19 the proportion of NBs with this condition increases to 4%.28
The estimated neonatal mortality rate before the COVID-19 pandemic was 18 cases per 1 000 live births (1.8%),84 and it was expected to increase due to the consequences of this disease. However, several reports14,16,30,45,85indicate that this rate is below 1%, and the percentage found in the present study ranges from 0% to 2.50%; furthermore, in another SR summary, Papapanou et al.45 found it to be 3%. These data suggest that neonatal mortality may have increased during the COVID-19 pandemic, but to corroborate this result, each region must be analyzed individually considering the socioeconomic disparities that affect this health indicator.
It should be noted that the degree of COVID-19 involvement of pregnant women is a conditioning factor with a high impact on maternal morbidity and neonatal mortality, as indicated in the preceding paragraphs. Jafari et al.28 were the only researchers to report that up to 80% of the newborns received breast milk substitutes (including mixed feeding), although they do not indicate whether this was a precautionary measure against SARS-CoV-2 transmission, or whether it was due to the degree of involvement of the mothers with COVID-19. However, the risk of transmission of the virus through breast milk has not yet been defined and appears to be low.69,86,87
The strengths of the present study were that it was conducted considering the best available evidence up to the date of writing, since it includes SRs from cohort and case-control studies. Moreover, the most reliable results were obtained by robust Bayesian analysis. On the other hand, it was limited by the fact that there are still very few SRs that include high quality primary cohort or case-control studies and most of them involve pregnant women in the second and third trimester, thus limiting the study of the teratological consequences that could be caused by the virus. Similarly, in the SR by Jafari et al.28 it was considered that there is a moderate risk of publication bias that could overestimate the severity and effects of the disease, as also recognized by the authors of the aforementioned study. Regarding the remaining SRs, it should be noted that the primary studies had different designs.
Conclusions
The most common symptoms in pregnant women with COVID-19 are cough, fever, dyspnea, and myalgia. Regarding complementary tests, the most frequent alterations are lymphopenia and lesions evidenced by x-ray or computed tomography of the thorax.
The presence of comorbidities in pregnant women with COVID-19 can lead to significant obstetric and neonatal complications, especially in symptomatic pregnant women or those with severe COVID-19 disease. It appears that SARS-CoV-2 infection in NBs is not severe and that the risk of vertical transmission is low, as no data on congenital malformations attributable to the virus were found. Given the nonspecificity of the symptoms, the physiological changes of pregnancy should be considered when assessing pregnant women.
The present review should be updated when new high-quality SRs with low risk of bias become available.