Introduction
Helicobacter Pylori is one of the most common bacterial infections worldwide, although it should be noted that most of the patients infected do not develop clinical manifestations of the disease.1
In a study on the origin of this bacterium, Linz et al. 2 describe that simulations indicate that H. pylori may have spread from East Africa about 58 000 years ago. Previously known as Campylobacter pyloridis, this microorganism was first described in 1982 by Marshall & Warren3 in patients with chronic gastritis, and since then it has been reported to occur in more than 50% of the world's population, being less frequent in developed countries.4,5 Currently, H. pylori is the main cause of chronic gastritis, peptic ulcers, mucosa-associated lymphoid tissue lymphoma (gastric MALT lymphoma), and gastric adenocarcinoma,6-9 and is considered a type 1 carcinogen.10
H. pylori infection is usually acquired during childhood and persists unless eradicated with antibiotics,6,8so clinical consensus suggests that eradication should be pursued once it has been identified.11-14The treatment for the eradication of this bacterium involves the use of triple or quadruple therapy. The former combines a proton pump inhibitor (PPI) with two antibiotics, namely, amoxicillin and clarithromycin if H. pylori resistance is <15% or amoxicillin and metronidazole if H. pylori resistance is <45%,11-13which is recommended as initial treatment for 14 days.11,12The latter combines a PPI with two antibiotics plus bismuth subsalicylate in 14-day schedules at varying doses and frequencies.
It has been suggested that the schemes chosen for the initial treatment of H. pylori infection should achieve an effectiveness of 95% on a per-protocol (PP) basis or at least 90% on an intention-to-treat (ITT) basis.11-14Nearly 20 years ago, triple therapy had an effectiveness of 90% or more, but today that effectiveness has declined to as low as 70%,9 which has been attributed primarily to antibiotic resistance.
In the last decade, it has been proposed that probiotics may be used as adjuvants in the eradication of H. pylori and the reduction of adverse effects secondary to triple or quadruple therapy used in the treatment of H. pylori infection. Increased eradication effectiveness has been reported mainly when probiotics are used in triple therapy, with an increase of 9-11%;15,16however, the same effect has not been reported when probiotics are used in quadruple therapy.17,18Thus, it is evident that the impact of probiotics on the eradication of H. pylori varies depending on the type of therapy used, which also leads to heterogeneity in their implementation in clinical practice due to the lack of consensus on their use.11,14
In view of the foregoing, the objective of the present systematic review and meta-analysis was to assess the effectiveness and safety of probiotics as adjuvants in triple or quadruple therapy for the eradication of H. pylori in adults. Likewise, as a secondary objective, it was proposed to explore the sources of heterogeneity that could explain the variable effect of probiotics depending on the therapy used that has been reported in the literature.
Materials and methods
Systematic review and meta-analysis aimed at answering the question: What is the effectiveness and safety of adjuvant probiotics in triple or quadruple therapy for the eradication of H. pylori in adults? This study followed the recommendations of the Cochrane Handbook for Systematic Reviews of Intervention19 and the PRISMA criteria.20
Search strategy
A systematic search was performed in Embase, Ovid Medline, Cochrane Library and LILACS using the following strategy: type of study: randomized clinical trials (RCTs) assessing the effectiveness and safety of using probiotics as adjuvant therapy in combination with triple or quadruple therapy for the eradication of H. pylori in adults; publication period: January 2010 to May 2020; language of publication: English and Spanish; search terms (alone and with different combinations and their variations depending on the database): "Helicobacter pylori", "H. pylori, eradication therapy", "triple therapy", "quadruple therapy, probiotics", "lactobacillus reuter", "Saccharomyces boulardii", "Lactobacillus plantarum", "Pediococcus acidilactici boulardii", "Lactobacillus rhamnosus", "Lactobacillus strains", "Lactobacillus bulgarius", "Streptococcus faecium", "Streptococcus thermophilus", and "Bifidobacterium longum", which were combined with the Boolean connectors "OR" and "AND" to establish the search equations.
Additionally, a gray literature search was performed in Google Scholar and the database search was supplemented by a snowball search.
Inclusion and exclusion criteria
Studies in adult patients (>18 years) in which the intervention group was treated with standard eradication therapy plus probiotics and the control group with the same eradication scheme with or without placebo were included. On the other hand, studies with pregnant women, trials using adjuvant eradication treatments other than probiotics, and papers whose full text was not available were excluded.
Two investigators (GJT and WAOR) independently performed the initial screening of the studies retrieved in the initial searches by reading titles and abstracts; subsequently, they verified compliance with the inclusion criteria by reading the full text. Disagreements regarding the final selection of studies were resolved by consensus.
Methodological quality and certainty of evidence
The methodological quality assessment of the selected studies was carried out based on the Cochrane Handbook for Systematic Reviews of Interventions,19 which evaluates the following domains: random sequence generation (selection bias), allocation concealment (allocation bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias). The risk of publication bias was assessed using funnel plots. The GRADE approach was used to assess the degree of certainty of the evidence supporting the interventions.21
Data extraction
Two reviewers extracted the data from the studies included in the review (GJT and KEO). During this process, general information about the studies was obtained, such as author/ authors, year and place of publication, design, and objectives. Likewise, the following information was extracted: characteristics and number of participants; types of standard therapy used (triple or quadruple); type of probiotics used; dose, frequency, and route of administration of probiotics; and quantitative data on the effectiveness and safety outcomes of adjuvant probiotics in combination with standard therapy for the eradication of H. pylori.
Statistical analysis
Data are presented descriptively using standardized tables, while information extracted from the studies included in the review is presented in summary tables. The pooled effect (odds ratio (OR) or relative risk (RR)) was estimated for the effectiveness (eradication) and safety (adverse events) outcomes, with their respective confidence intervals (95%CI) depending on the type of outcome. Absolute risk reduction and relative risk reduction of the assessed interventions versus placebo or no intervention were also calculated.
The statistical model to determine the pooled effect using OR and RR was selected based on the number of included studies and their heterogeneity, which was tested during the analysis using the chi-square test (x2) and the index of inconsistency (I2), where heterogeneity values >30% measured with I2 but with p-values >0.05 obtained using x2 showed that the differences in effect can be explained by chance. Given the foregoing, a fixed effects model for the meta-analysis and the Z value for the statistical significance test were performed to estimate the pooled measure of effect.
Subgroup analyses were also performed considering the type of eradication therapy (triple or quadruple) and the type of probiotic used. Moreover, clinical, statistical, and methodological sources of heterogeneity between studies were explored by means of a sensitivity analysis when heterogeneity according to P was >60%. When it was not possible to explain the high heterogeneity (P>60%) or when it was not possible to adjust by presenting the evidence by subgroups, the range of effects was presented per type of outcome based on the estimator reported in the studies, together with their respective 95%CI. Statistical analyses were performed in RevMan software.
Results
Identified and selected studies
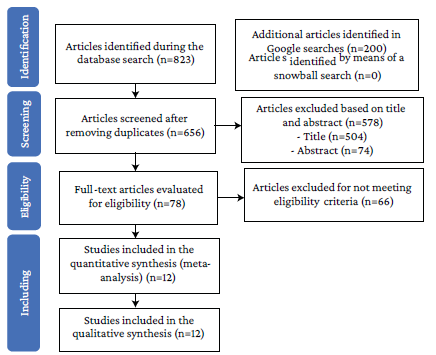
The initial search yielded 1 023 results, 823 from the databases and 200 from Google Scholar, of which 367 were removed because they were duplicates and 578 after reviewing the title and abstract (504 and 74, respectively). Of the 78 references selected for full-text review, 21 were excluded because the full text was not available, 11 because they were written in languages other than English or Spanish, 15 because they were not RCTs, and 16 because they did not meet the inclusion criteria; there were also 3 duplicate studies (details of the excluded studies are presented in Annex 1). Thus, 12 studies were included for full analysis (Figure 1).
The 12 studies included in the meta-analysis are RCTs, and of these, 10 were double-blind,22-311 was triple-blind,32 and in the other no placebo was used.33 Triple therapy was used in 9 of these 12 studies22-25,27-30,33with a total of 824 participants, while quadruple therapy was used in 2,31,32with a total of 256 participants; similarly, one of these studies evaluated the use of both triple therapy and quadruple therapy.26
Studies on triple therapy
Of the 9 studies that used triple therapy, 2 used the drugs clarithromycin, amoxicillin, and lansoprazole.;24,28 6 used a triple therapy with different drugs: omeprazole plus clarithromycin and amoxicillin,27 rabeprazole plus clarithromycin and tinidazole,22 esomeprazole plus levofloxacin and amoxicillin,33 lansoprazole plus tetracycline and furazolidone,29clarithromycin plus amoxicillin and rabeprazole,30 and esomeprazole plus amoxicillin and clarithromycin;23 finally, a single study used clarithromycin and amoxicillin and did not specify the PPI.25
The duration of triple therapy was variable: in 4 studies, probiotics were administered for 2 weeks;24,27,30,33in 4 studies, they were administered for 4 weeks;22,23,28,29and in 1 study, they were administered for 13 weeks.25
Studies on quadruple therapy
Both studies that analyzed quadruple therapy31,32used bismuth, amoxicillin, clarithromycin, and omeprazole for 2 weeks.31,32
Triple therapy and quadruple therapy studies
The study that evaluated the use of both therapies used omeprazole, clarithromycin and amoxicillin for 10 days in the triple therapy and bismuth, amoxicillin, clarithromycin and omeprazole, also for 10 days, in the quadruple therapy.26
Probiotics as an adjuvant therapy
Of the 12 studies included in the analysis, 4 used Lactobacillus reuteri as an adjuvant probiotic,22,25,27,33 5 used a mixture of probiotics,26,28,29,31,321 used Saccharomyces, 24 another used Bacillus clausi, 30 and another used Bifidobacterium longum. 23
In 11 studies, a comparison versus placebo plus triple or quadruple therapy was made, except in the study by Ojetti et al., 33 where the comparison was made only with triple therapy. Likewise, 10 studies specified that the placebo was equal to the investigational product in terms of form, presentation, smell and taste,22-31while the other did not specify anything in this regard.32 Details of the included studies are presented in Tables 1 and 2.
Table 1 Overall characteristics of the included studies.
| Study | Methods | Participants | Type of therapy | Intervention/dose/ duration | Control | Outcomes |
|---|---|---|---|---|---|---|
| Armuzzi et al. 22 2001 Italy | Double-blind, prospective, randomized, placebo-controlled trial | Healthy asymptomatic patients over 18 years of age, positive for Helicobacter pylori and under treatment | Triple therapy: rabeprazole, clarithromycin and tinidazole | Lactobacillus GG for 14 days | Placebo | - Adverse events - Eradication |
| Chitapanarux et al. 23 2015 Thailand | Double-blind, prospective, randomized, placebo-controlled trial | Patients with H. Pylori infection on antibiotic treatment | Triple therapy: esomeprazole, amoxicillin and clarithromycin | Bifidobacterium longum for 4 weeks | Placebo | - Adverse events - Eradication |
| Cindoruk et al. 24 2007 Turkey | Prospective, randomized, placebo-controlled trial | Adult patients with H. pylori infection confirmed on upper digestive tract biopsy and dyspepsia symptoms | Triple therapy: clarithromycin, amoxicillin and lansoprazole | Saccharomyces boulardii for 14 days | Placebo | - Adverse events - Eradication |
| Francavilla et al. 25 2014 Italy | Double-blind, prospective, randomized, placebo-controlled trial | Adult patients (over 18 years of age) with dyspepsia who had never received H. pylori eradication therapy | Triple therapy: proton pump inhibitor, clarithromycin and amoxicillin | Lactobacillus reuteri (mixture of L. reuteri DSM 17938 and L. reuteri ATCC PTA 6475) for 28 days | Placebo | - Adverse events - Eradication |
| Emara et al. 27 2014 Egypt | Double-blind, prospective, randomized, placebo-controlled trial | Adult patients (18-60 years old) with dyspepsia and confirmed H. pylori infection and no previous treatment | Triple therapy: omeprazole, amoxicillin and clarithromycin | L reuteri (mixture of L. reuteri DSM 17938 and L. reuteri ATCC PTA 6475) for 4 weeks | Placebo | - Adverse events - Eradication |
| Myllyluoma et al. 28 2005 Finland | Double-blind, randomized, placebo-controlled trial | Adult patients (18-70 years of age) with H. pylori infection confirmed by rapid blood test | Triple therapy: lansoprazole, clarithromycin and amoxicillin | Lactobacillus rhamnosus GG, L. rhamnosus LC705, Bifidobacterium breve Bb99, and Propionibacterium freudenreichii ssp. shermanii JS for 3 weeks | Placebo | - Adverse events - Eradication |
| Navarro-Rodríguez et al. 29 2013 Brazil | Double-blind, randomized, placebo-controlled trial | Adult patients (over 18 years of age) with previously untreated H. pylori infection, diagnosed with peptic ulcer or functional dyspepsia, without decompensated chronic disease, and no use of antiinflammatory drugs or antibiotics in the last 4 weeks | Triple therapy: lansoprazole, tetracycline and furazolidone | Lactobacilos acidophilus, L. rhamnosus, Bifidobacterium bifidum, and Streptococcus faecium for 30 days | Placebo | - Adverse events - Eradication |
| Nista et al. 30 2004 Italy | Single-center, double-blind, prospective, randomized, placebo- controlled trial | Adult patients (18-65 years old) without gastrointestinal symptoms in the last 3 months and with H. pylori infection confirmed by breath test | Triple therapy: rabeprazole, clarithromycin, amoxicillin | Bacillus clausii and Enterogermina for 14 days | Placebo | - Adverse events - Eradication |
| Ojetti et al. 33 2012 Italy | Single-center, controlled, prospective, randomized trial | Adult patients (18-65 years old) with H. pylori infection confirmed by breath test | Triple therapy: esomeprazole, levofloxacin and amoxicillin | L. reuteri for 14 days | None | - Adverse events - Eradication |
| Shafaghi et al. 31 2016 Iran | Double-blind, prospective, randomized, placebo-controlled trial | Patients with H. pylori infection confirmed by histopathological examination | Quadruple therapy: bismuth, clarithromycin, amoxicillin and omeprazole | Lactobacillus casei, L. rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, L. acidophilus, B. longum, and Lactobacillus bulgarius for 14 days | Placebo | - Adverse events - Eradication |
| Shavakhi et al. 32 2013 Iran | Triple-blind, randomized, placebo-controlled trial | Adult patients with peptic ulcer disease and H. pylori infection confirmed by rapid urease test or histological studies | Quadruple therapy: omeprazole, bismuth, amoxicillin and clarithromycin. | Seven bacterial species including Lactobacillus strains (L. casei, L. rhamnosus, L. acidophilus and L. bulgaricus), Bifidobacterium strains (B. breve and B. longum), and Streptococcus thermophiles | Placebo | - Adverse events - Eradication |
| McNicholl et al. 26 2018 Spain | Double-blind, randomized, placebo-controlled trial | Adult patients (18-70 years of age) with confirmed H. pylori infection | Triple therapy: omeprazole, clarithromycin and amoxicillin Quadruple therapy: clarithromycin, bismuth, amoxicillin and omeprazole | Lactobacillus plantarum CETC7879 and Pediococcus acidilactici CETC7880 for 10 days | Placebo | - Adverse events - Eradication |
ATCC: American Type Culture Collection, Manassas, VA, USA. DSM: DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany.
Source: Own elaboration.
Table 2 General characteristics of the studies.
| Study | Number of patients | Probiotics | Control | Age (mean) | Female sex and probiotics | Female sex control (n) | Male sex and probiotics | Male sex control (n) | Eradication with probiotics | Eradication control (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| Armuzzi et al. 22 2001 | 60 | 30 | 30 | 40 years old | NR | NR | NR | NR | 25 | 24 |
| Chitapanarux et al. 23 2015 | 63 | 31 | 32 | NR | 17 | 18 | 14 | 14 | 28 | 22 |
| Cindoruk et al. 24 2007 | 124 | 62 | 62 | 48 years old | 36 | 44 | 26 | 18 | 44 | 37 |
| Francavilla et al. 25 2014 | 86 | 43 | 43 | NR | 32 | 29 | 18 | 21 | 33 | 29 |
| McNicholl et al. 26 2018 | 68 | 34 | 34 | NR | NR | NR | NR | NR | 32 | 31 |
| Emara et al. 21 2014 | 70 | 35 | 35 | NR | 22 | 24 | 13 | 11 | 26 | 23 |
| Myllyluoma et al. 28 2005 | 47 | 24 | 23 | 55.6 years old | 13 | 16 | 10 | 8 | 21 | 19 |
| Navarro-Rodríguez et al. 29 2013 | 107 | 52 | 55 | NR | 34 | 33 | 21 | 19 | 45 | 40 |
| Nista et al. 30 2004 | 120 | 60 | 60 | NR | 27 | 35 | 33 | 25 | 39 | 37 |
| Ojetti et al. 33 2012 | 90 | 45 | 45 | NR | NR | NR | NR | NR | 36 | 27 |
| Shafaghi et al. 31 2016 | 76 | 38 | 38 | 43.5 years old | 20 | 21 | 18 | 17 | 35 | 24 |
| Shavakhi et al. 32 2013 | 180 | 90 | 90 | NR | 41 | 30 | 49 | 60 | 73 | 69 |
NR: not reported; n: absolute number of participants.
Source: Own elaboration.
Bias assessment
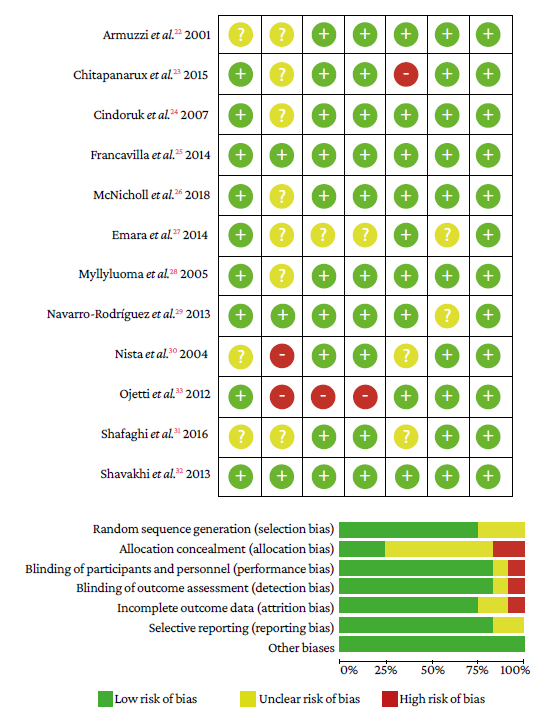
The risk of random sequence generation (selection bias) was unclear in 3 studies.22,30,31In the allocation concealment domain, 2 studies had high risk30,33and the risk was unclear in 7.22-24,26-28,31in the domains blinding of participants and personnel, and blinding of outcome assessment, 1 study showed high risk33 and in another the risk was unclear.27 In the incomplete outcome data domain, 1 study showed high risk23 and the risk was unclear in 2 studies.30,31Finally, in the selective reporting domain, the risk was not clear in 2 studies.27,29No other types of bias were observed in the studies (Figure 2).
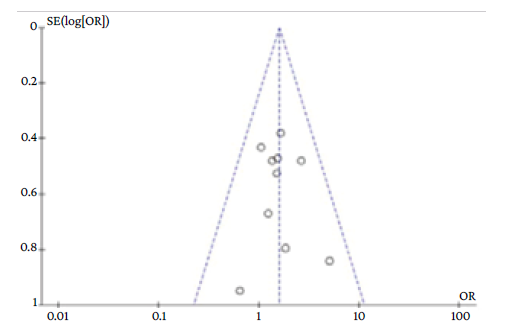
The exploration of publication bias in the funnel plot is reported in Annex 2.
Outcomes
H. pylori eradication
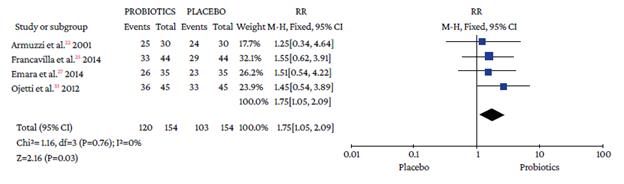
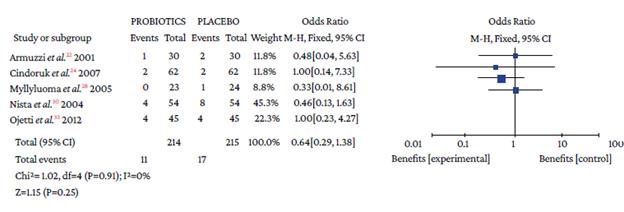
The use of probiotics as adjuvant therapy in triple therapy for H. pylori eradication showed a benefit over the use of placebo (OR=1.48; 95%CI: 1.05-2.09; I2=0%), as illustrated in Figure 3, which also reports the x2 and I2 heterogeneity values and the Z value of the statistical significance test for the pooled measure of effect.
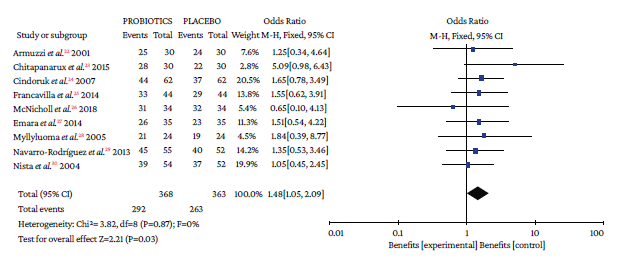
Source: Own elaboration.
Figure 3 Pooled effect measure for Helicobacter pylori eradication outcome in triple therapy. Events: number of H. pylori eradication events; Total: number of participants in each group (triple therapy plus probiotics is labeled as probiotics and triple therapy plus placebo as placebo).
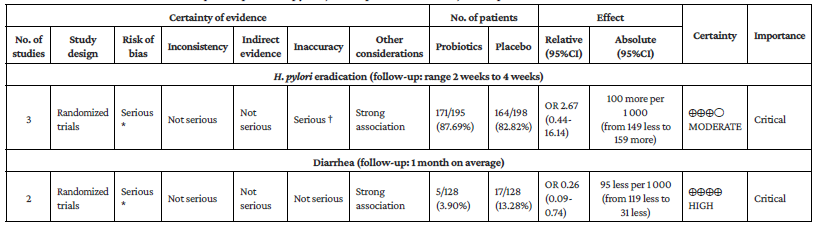
Regarding the use of adjuvant probiotics in quadruple therapy, it was not possible to estimate a pooled effect measure due to the high heterogeneity (P=78%); however, in the three studies identified, there was no evidence of differences in the effect found between the use of probiotics and placebo as adjuvant therapy: (OR=7, 95%CI: 0.35-138.14),26 (OR=6.81, 95%CI: 1.76-26.27),31 and (OR=0.77, 95%CI: 0.37-1.57).32 Moreover, an absolute and relative increase in H. pylori eradication success with probiotics versus placebo of 8.3% and 11%, respectively, was found. Likewise, adjuvant therapy with the probiotic L. reuteri plus quadruple therapy showed a significant benefit compared to placebo (RR=1.75, 95%CI: 1.05-2.92; P=0 %), with an absolute eradication rate of the probiotic group exceeding the eradication treatment by 5.51% and a relative increase in eradication rate success of 14.16% (Annex 3).
In the relative risk analysis, it was observed that 2 studies using a probiotic mixture did not show a significant benefit in terms of effectiveness compared to the use of placebo: (RR=0.969, 95%CI: 0.847-1.108)26 and (RR=0.945, 95%CI: 0.812-1.100)32 (Table 3).
Table 3 Assessment by probiotic in triple and quadruple therapy.
| Study | Relative risk reported in the original study | 95%CI | Risk difference | 95%CI |
|---|---|---|---|---|
| Probiotic mixture | ||||
| McNicholl et al. 26 2018 | 0.969 | 0.847-1.108 | -0.029 | (-0.153)-0.094 |
| Myllyluoma et al. 28 2005 | 1.105 | 0.857-1.426 | 0.083 | (-0.127)-0.293 |
| Navarro-Rodríguez et al. 29 2013 | 1.064 | 0.876-1.292 | 0.049 | (-0.104)-0.202 |
| Shafaghi et al. 31 2016 | 1.458 | 1.124-1.891 | 0.289 | 0.114-0.465 |
| Shavakhi et al. 32 2013 | 0.945 | 0.812-1.100 | -0.044 | (-0.163)-0.075 |
| Saccharomyces | ||||
| Cindoruk et al. 24 2007 | 1.189 | 0.918-1.541 | 0.113 | (-0.053)-0.279 |
| Bacillus clausii | ||||
| Nista et al. 30 2004 | 1.015 | 0.799-1.290 | 0.010 | (-0.161)-0.182 |
| Bifidobacterium longum | ||||
| Chitapanarux et al. 23 2015 | 1.314 | 1.013-1.705 | 0.216 | 0.024-0.407 |
CI: confidence interval.
Source: Own elaboration.
Safety profile: adverse events
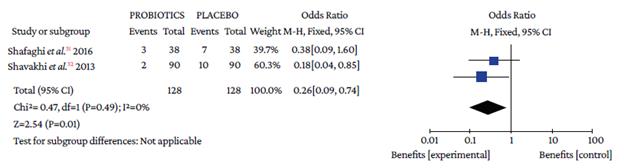
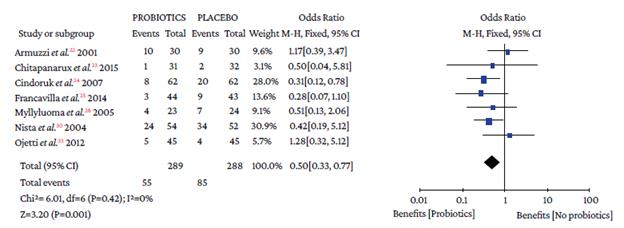
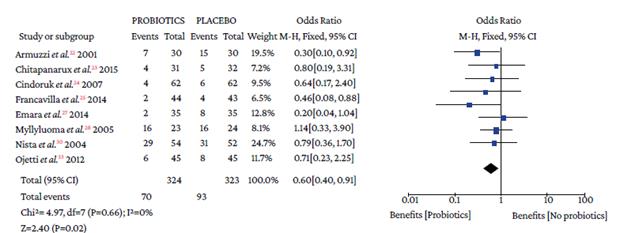
The use of probiotics in combination with triple therapy was associated with lower overall adverse effects compared to placebo (OR=0.50, 95%CI: 0.28-0.90; P=0%), which also occurred in combination with quadruple therapy (OR=0.26, 95%CI: 0.09-0.74; I2=0%) (Figure 4).
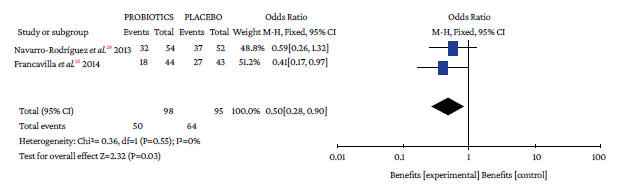
Events: number of overall adverse events; Total: number of participants in each group (triple therapy plus probiotic is labeled as probiotics and triple therapy plus placebo as placebo).
Source: Own elaboration.
Figure 4 Adverse events with triple therapy.
When analyzing the occurrence of adverse events in triple and quadruple therapy, it was found that the absolute effect of any adverse event in the probiotics group was lower by 16.4% and 9.37%, respectively. Likewise, the absolute and relative reduction in the risk of suffering any adverse event with probiotics plus triple therapy was 16.34% and 24.26%, respectively (Table 3).
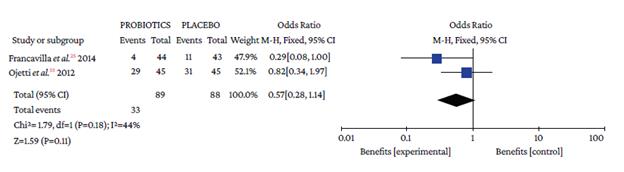
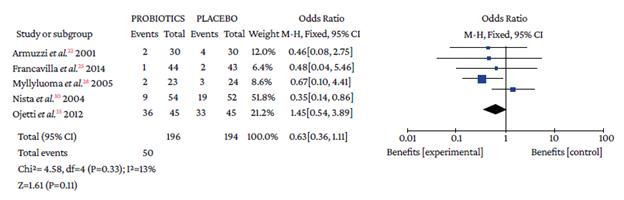
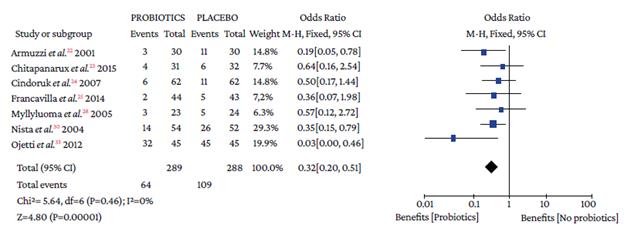
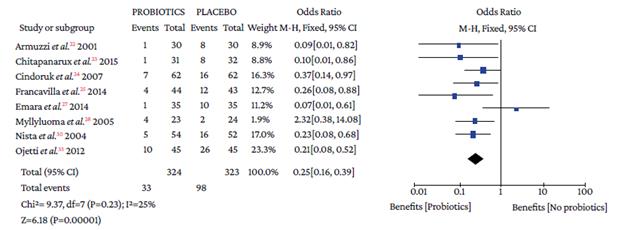
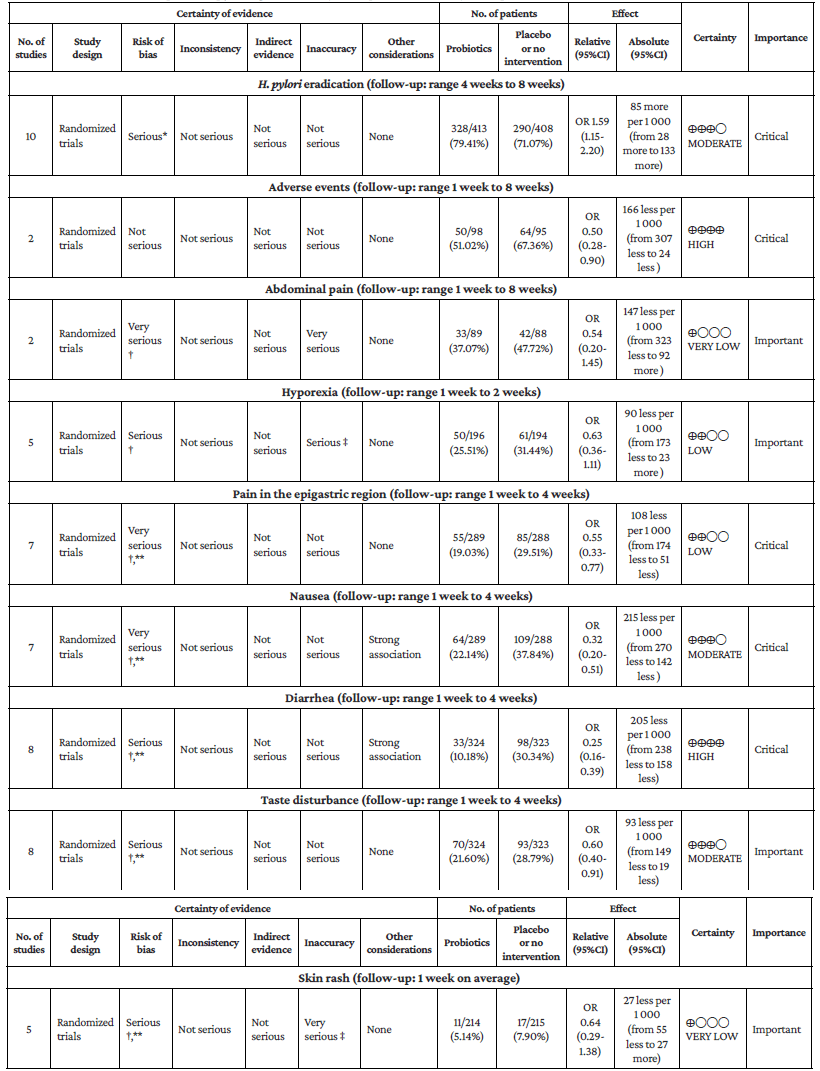
Regarding the type of adverse event reported, no differences were found with the use of probiotics plus triple therapy compared to placebo in relation to abdominal pain (OR=0.57, 95%CI: 0.28-1.14; P=44%), anorexia (OR=0.63, 95%CI: 0.36-1.11; P=13%), or rash (OR=0.64, 95%CI: 0.29-1.38; P=0%) (Annex 3). However, in the group with probiotics, a lower occurrence of pain in the epigastric region (OR=0.50, 95%CI: 0.33-0.77; P=0%), nausea (OR=0.32, 95%CI: 0.20-0.51; P=0%), diarrhea (OR=0.25, 95%CI: 0.16-0.39), and taste disturbance (OR=0.60, 95%CI: 0.40-0.91) was observed compared to the placebo group (Annex 3).
Assessment of evidence certainty
The evidence certainty assessment (GRADE approach) for patients receiving triple and quadruple therapy found that the certainty of the evidence evaluating the eradication of H. pylori with probiotic adjuvant triple therapy was moderate, while the certainty of the evidence for the occurrence of adverse events such as diarrhea, pain in the epigastric region, hyporexia, rash, among others, ranged from very low to moderate. In the case of H. pylori eradication with adjuvant probiotics in quadruple therapy, the certainty of the evidence was moderate, but when evaluating the decrease in adverse events such as diarrhea, the certainty of the evidence was high (Annex 4).
Discussion
In the present review, which looked into the effectiveness and safety of adding probiotics to conventional treatment for H. pylori eradication, it was found that, compared to the use of placebo, adding probiotics to triple therapy increased the eradication rate by 8.3%. This increase is similar to the one reported in other meta-analyses such as that of Gong et al., 34 which included 23 randomized clinical trials involving 3 900 subjects receiving triple therapy and found that the addition of probiotics to this therapy increased eradication by 8.48% (72.26% vs. 80.74%); Zhang et al., 35 which included data from 6 997 participants from 45 randomized controlled trials and showed that adding probiotics to standard therapy increased the eradication rate by 10.23% over placebo (82.31% vs. 72.08%); and the double-blind, randomized, placebo-controlled trial conducted by Dore et al., 16 who analyzed 56 subjects with a low cure rate and found that the effectiveness rate in the probiotics group was 12.5% for PP and 10.7% for ITT. The meta-analysis carried out by Lau et al., 15 in which 30 RCTs with a total of 8 817 patients were analyzed, also reported comparable results: the eradication rate increased by 12.2% when probiotics were added to triple therapy.
The present meta-analysis found that the most commonly used probiotic was L. reuteri, which was evaluated in 4 studies (154 patients in total).22,25,27,33The eradication rate with this microorganism was 77.9% (95%CI: 70.5-84.19), while placebo showed 66.8% (95%CI: 58.8-74.2), which is consistent with the results reported by Dore et al. 16 who compared the use of L. reuteri (Gastrus®) plus PPI versus placebo plus PPI, finding that the eradication of H. pylori was higher with the first combination (11% vs. 3.6%).
Although there was evidence of an increase in the effectiveness of H. pylori eradication with the use of probiotics in combination with triple therapy, the effectiveness of the intervention measured as eradication did not exceed 90% for ITT or 95% for PP, which are the minimum thresholds required for eradication therapies for this bacterium.11-14Therefore, using empirical triple therapies with or without probiotics to eradicate H. pylori is not justified.
Unlike triple therapies, the present meta-analysis found that adding probiotics to quadruple therapies does not increase the effectiveness to eradicate H. pylori compared to placebo, which is 87.7% vs. 82.8% (OR=2.67, 95%CI: 0.44-16.4%; P=78%). However, it should be kept in mind that the low statistical power of the individual studies and the high heterogeneity among the included studies (I2=78%) hindered a pooled analysis.
Another hypothesis that could explain why there was no increase in the effectiveness of quadruple therapy with bismuth is the effect that bismuth would have on inhibiting the growth of probiotics.16 In this regard, Dore et al. 18 report, based on two randomized open pilot studies with 46 patients each, that a 10-day quadruple therapy (bismuth, teracy-cline, metronidazole, and PPI) had an effectiveness of 95.7% for PP (95% CI: 85-99%) and 84.8% for ITT (95% CI: 71-95%), and that patients treated with Gastrus® (BioGaia, Stockhplm, Sweden) showed an effectiveness of 79.6% for PP (95%CI: 72-97) and 84.8% for ITT (95%CI: 71-95). In the subgroup analysis, quadruple therapy with bismuth was 100% effective (25/25) in naive patients versus 90% (26/29) in those treated with Gastrus®. A drawback of the study by Dore et al. 18 is that it did not establish the susceptibility of H. pylori to the antibiotics used.
Concerning adverse effects, in the present study, an overall reduction of 16.34% was found for any adverse effect in triple therapies when probiotics were added, while the reduction in quadruple therapies was 9.37%. The individual analysis of adverse effects found that there was no difference between probiotics and placebo for the incidence of abdominal pain, anorexia or rash in triple therapies, although there was a lower incidence of pain in the epigastric region, nausea, diarrhea, and taste disturbance with the use of probiotics. However, it was not possible to determine whether the decrease in adverse effects improved treatment compliance.
Although there are other meta-analyses that assess the effectiveness of probiotics as an adjuvant to standard therapies for H. Pylori eradication, the present study has been conducted by subgroups of interest to establish the effectiveness of triple therapy plus probiotics when compared to triple therapy with placebo and no intervention, as well as the effectiveness of eradication therapy plus probiotics differentiated by type of probiotic, finding a moderate certainty of evidence to support this outcome, which ensures confidence in the results presented here.
In the case of quadruple therapy, this review has some limitations secondary to the low number of included studies comparing the adjuvant use of this type of therapy in combination with probiotics or placebo, which translates into a low statistical power that could not be corrected with pooled analyses given the high heterogeneity of the effects reported in these studies. In this regard, further studies are needed to evaluate whether adding probiotics to quadruple therapy increases its effectiveness in eradicating H. pylori.
Conclusion
The findings of the present study demonstrate that the adjuvant use of probiotics increases the effectiveness of triple therapy to eradicate H. pylori by 8.5%, but that the final effectiveness was not 90-95% in any case, which is the minimum threshold required in eradication therapies for this microorganism. Regarding quadruple therapy, it was established that probiotics do not modify its effectiveness, although there is uncertainty in the results given the inaccuracy of the effect estimators.
Concerning the safety of probiotics in combination with triple or quadruple therapy for H. pylori eradication, a lower incidence of adverse effects was found, although it could not be established whether this decrease favors adherence to treatment.
Based on the results obtained, it can be concluded that it is not necessary to continue studying the effect of adding probiotics to triple therapies for the eradication of H. pylori, but it is advisable to carry out new studies to evaluate this effect in quadruple therapies.
Explanatory note
This publication is derived from the thesis entitled Efectividad y seguridad del uso de probióticos en la erradicación de Helicobacter pylori: revisión sistemática de la literaturay meta-análisis (Effectiveness and safety of the use of probiotics in the eradication of Helicobacter pylori: systematic literature review and meta-analysis),36 developed by the authors of this manuscript for obtaining a degree in gastroenterology.