Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.3 Bogotá July/Sept. 2017
https://doi.org/10.22516/25007440.156
Review articles
Small Bowel Bleeding: Approach and Treatment
1Residente de medicina interna, Universidad Nacional de Colombia, Hospital Universitario Nacional de Colombia, Bogotá (Colombia).
2Internista, fellow de gastroenterología, Universidad Nacional de Colombia, Hospital Universitario Nacional de Colombia, Bogotá (Colombia).
3Profesor titular de Medicina, Universidad Nacional de Colombia; coordinador de gastroenterología, Hospital Universitario Nacional de Colombia; gastroenterólogo, Clínica Fundadores, Bogotá (Colombia). Correo electrónico: waoteror@gmail.com
Intestinal lesions that were previously inaccessible can now be identified. The most important new diagnostic tool is the endoscopic videocapsule because of its high negative predictive value. With advances in endoscopic methods, the classification of gastrointestinal bleeding has changed so that definition of occult and obscure bleeding that previously included bleeding originating in the small intestine has been relegated to cases for which the origin cannot be identified after performing esophagogastroduodenoscopy, colonoscopy and studies of the middle digestive tract such as endoscopic videocapsule, push enteroscopy, deep enteroscopy , intraoperative enteroscopy, MRI enterography, CT enterography, angiography and scintigraphy.
Keywords: Small intestinal hemorrhage; obscure gastrointestinal bleeding; endoscopic videocapsule; enteroscopy; enterography
Actualmente, se pueden identificar lesiones del intestino delgado que antes eran inaccesibles. La principal herramienta diagnóstica es la videocápsula endoscópica por el alto valor predictivo negativo. Con los avances en los métodos endoscópicos, la clasificación del sangrado gastrointestinal ha cambiado. Es así como la definición del sangrado oscuro, que antes incluía al originado en el intestino delgado, se ha relegado cuando su origen no se puede identificar tras la realización de una esofagogastroduodenoscopia, colonoscopia y estudios del tracto digestivo medio, tales como videocápsula endoscópica, enteroscopia de empuje, enteroscopia profunda, enteroscopia intraoperatoria, enterorresonancia, enterotomografía, angiografía y gammagrafía.
Palabras claves: Hemorragia de intestino delgado; sangrado gastrointestinal oscuro; videocápsula endoscópica; enteroscopia; enterografía
Introduction
Traditionally, digestive bleeding has been classified into upper gastrointestinal bleeding (UGB) and lower gastrointestinal bleeding (LGB). If bleeding originates above the ligament of Treitz, it is UGB, and if it occurs in the colon from the cecum to the anus it is LGB. 1 However, the source of bleeding cannot be identified with upper digestive tract endoscopy or colonoscopy in 10% to 20% of patients. 2. The traditional diagnosis of these cases was “bleeding of obscure origin”. At present, the diagnosis is “probable small bowel bleeding” which requires further study with methods that evaluate the small intestine or which may require repetition of upper endoscopy or colonoscopy depending on the initial scenario. 3 Repetition of upper endoscopy is recommended when there is recurrent hematemesis or melena, and repetition of colonoscopy is recommended when there is recurrent hematochezia. 4) The cause of bleeding is identified in 2% to 25% of cases by the second upper endoscopy, and in 6% to 23% by the second colonoscopy. 5 Current methods of evaluating the small intestine show that between 5% and 10% of all gastrointestinal bleeding originate in this segment and up to 75% of all bleeding previously considered to be of obscure origins originate there. 6
Anatomically, small intestine bleeding includes that which occurs at any site from the Ampulla of Vater to the ileocecal valve. Bleeding in this segment has also been called “bleeding of intermediate origin”. 7 The small intestine is between six and seven meters m long and is about 2.5 cm in diameter. It consists of the duodenum (30 cm), the jejunum (250 cm) and the ileum (350 cm). 8 Due to its length and anatomical disposition, it had been a difficult organ to study. However, with the advent of new technologies, it can now be evaluated. 9 When the origin of bleeding is not found after examining all the segments mentioned, the diagnosis is “gastrointestinal bleeding of obscure origin” as summarized in Figure 1. 10
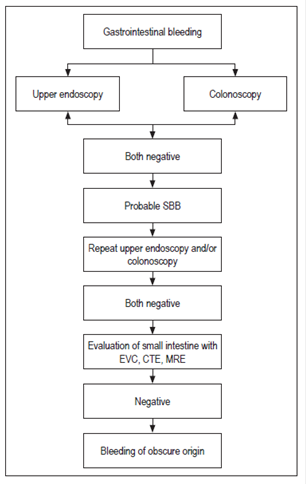
Figure 1 Definition of probable small bowel bleeding and bleeding of obscure origin. EVC: endoscopic videocapsule; CTE: CT Enterography; MRE: MR enterography
Methods available for study of the small intestine include push enteroscopy, balloon-assisted enteroscopy, spiral enteroscopy, endoscopic videocapsule (EVC), computed tomography enterography, magnetic resonance enterography and intraoperative enteroscopy. 11. Intraoperative enteroscopy is considered to be the “gold standard” for studies of the small intestine. Other complementary methods for locating the origin of bleeding are arteriography, magnetic resonance angiography, CT angiography and scintigraphy. 12
Methodology
The following MeSH terms and keywords were used in the search strategy for this study: small bowel bleeding, gastrointestinal bleeding, obscure bleeding, occult bleeding, overt bleeding, capsule endoscopy, single-balloon enteroscopy, double-balloon enteroscopy, push enteroscopy, spiral enteroscopy, angiography, iron-deficiency anemia, magnetic resonance enterography, Meckel’s diverticulum, deep enteroscopy, intraoperative enteroscopy, CT enterography, scintigraphy. The search was limited to studies conducted in humans published in English and Spanish from 2005 to October 2016. The electronic databases investigated were Cochrane, Central Controlled Trials, MEDLINE, EMBASE and Science Citation Index. We also searched manually. After identification of publications, the authors chose those that they believed to be the most relevant.
Clinical manifestations
A patient has “potential bleeding” from the small intestine when upper endoscopy and colonoscopy are negative. Clinically, bleeding may be perceptible as in melena or hematochezia (70%), or it can be occult (30%). The latter is identified by iron-deficiency anemia or a positive test for fecal occult blood. 13
Etiology of small bowel bleeding (SBB)
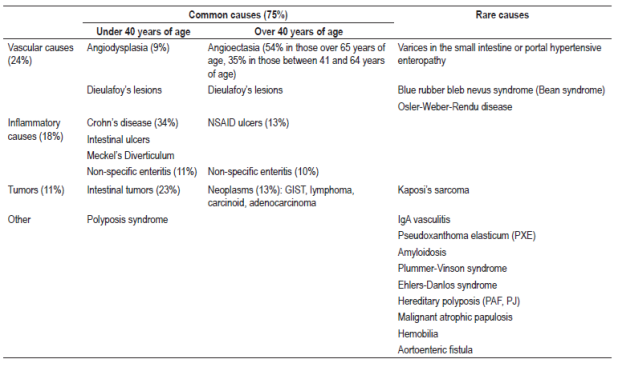
There are numerous causes of SBB including vascular, inflammatory and tumor origins. The different causes vary with age (Table 1) and geographical areas. In South Korea, ulcers (26%), angiodysplasias (10%), erosions (8%) and tumors of the small intestine (2%) have been found to be the most frequent causes. 14 In Western countries, 70% are vascular, and angioectasias are the most frequent (20% to 55%), followed by tumors (10% to 20%) and Crohn’s disease (2% to 10%). 15
Clinical approach
The approach to patients with SBB starts with a good clinical history including complete anamnesis and physical examination. The bleeding is rarely severe, 16 but when it is, the priority is hemodynamic stabilization of the patient. 17 SBB can clinically manifest as melena or hematochezia depending on the extent of bleeding and intestinal transit. Consequently, these forms are not useful for predicting the origin of bleeding. 18 Comorbidities should be investigated. These include von Willebrand disease; valvular heart disease; hemodialysis; portal hypertension; vasculitis; amyloidosis; use of aspirin, NSAIDs or anticoagulants; previous procedures including liver biopsies, liver transplantation, repairs of abdominal aortic aneurysms, intestinal resection and radiation therapy. Other factors that should be investigated include family histories of inflammatory bowel disease, polyposis, malignant diseases and familial telangiectasia. 19 Older adults with valvular heart disease, chronic kidney disease or connective tissue diseases are at high risk of having vascular lesions in the small intestine at risk of bleeding. During the physical examination, the physician should look for predisposing pathologies such as hereditary hemorrhagic telangiectasia (telangiectasias on the lips or oropharynx), Kaposi’s sarcoma (dark violaceous macules on skin and mucous membranes), Peutz-Jeghers syndrome (dark brown macules on the mucosa of the mouth and around the lips), elastic pseudoxanthoma (yellowish papules that can come together to form plaques on the neck, elbows, popliteal fossae and umbilical region), Ehlers-Danlos syndrome (hyperlaxity and papyrus scars), Bean syndrome (blue nodules), IgA vasculitis (palpable purpura) and neurofibromatosis (café-au-lait spots and subcutaneous neurofibromas). 20
Diagnostic methods
Current technology has overcome the limitations of traditional radiological tests. The indications, advantages and limitations of the different modalities for evaluating the small intestine are discussed below.
Endoscopic Videocapsule (EVC)
Endoscopic Videocapsules (EVC) are considered to be the exam of choice for examining the small intestine. They are digestible and disposable devices that measures 26 × 11 mm and which are generally expelled within eight to 72 hours. 21 The capsule contains a video camera, a light source, a radio transmitter and batteries. It take two pictures/second for 8 to 12 hours. The images are transmitted to a recorder that is located in the abdomen of the patient. The data stored in the recorder are uploaded to a computer that has a specific software for analysis. 22 EVC was created in 1981 and introduced in the United States in 2001. It evaluates the entire small intestine in 79% to 90% of patients. Its diagnostic performance is 38% to 83%, its sensitivity is 95%, its specificity of is 75%, and it has a positive predictive value of 94% to 97% and a negative predictive value of 83% to 100%. 23.
The probability of finding lesions by this method correlates positively when there is less than 10 g% hemoglobin, duration of bleeding is greater than 6 months, there is been more than one episode of bleeding, bleeding is overt rather than occult (60% vs. 46% , respectively). 24 Performance is also better in men, in people who are over 60 years of age, in hospitalized patients, in cases in which the examination is performed within two weeks of the episode of bleeding (91% vs. 34%), and when there are cardiac and/or renal comorbidities. 25 Maximum performance is achieved when the test is performed between 48 and 72 hours after bleeding. 26
Prior to the study, the patient should be prepared as for a colonoscopy. The current recommendation is to use polyethylene glycol (PEG) diluted in 2 liters of water. This can be used alone or in combination with simethicone to guarantee better quality preparation and higher diagnostic yield. 27 Two hours after the start of the study, the patient can drink liquids. Five hours after the start, the patient can eat normally. 28 Although most patients swallow the capsule without difficulty, for patients with swallowing disorders, it is necessary to endoscopically place the capsule directly into the stomach or small intestine. Special accessories can be used to advance the capsule with the endoscope when required. These include the AdvanCE, polypectomy loops (to grasp it) and overtubes. 29
The only formal contraindication is suspicion of intestinal obstruction which could cause the capsule to be retained potentially resulting in the necessity of surgical removal. 30 Contraindications regarding pregnancy, cardiac assist devices, diabetic gastroparesis, dementia and Zencker’s diverticula have been considered. 31
Advantages
Endoscopic videocapsules are well tolerated by patients, are not invasive, and produce minimal discomfort. They can examine the entire small intestine to help determine whether shortest route to reach a lesion with complementary enteroscopy is through the mouth (anterograde) or through the anus and then through the ileocecal valve (retrograde). 32 Depending on the type of lesion found, it can be treated with enteroscopy, angiography or surgery.
Limitations
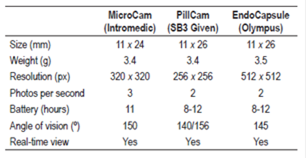
Limitations include inability to control their movement through the gastrointestinal tract, difficulty in determining the location of the lesion, low specificity (14% of incidental findings in healthy volunteers) and false negatives in 10% to 36% of cases. 33 Also, they cannot enter the small intestine when there is a Roux-en-Y reconstruction and provide limited views of diverticula. 34 In most cases, the papilla cannot be identified and their performance is poor for lesions of the duodenum and proximal jejunum. They also have limited capacity to detect small submucosal tumors and produce false negatives in up to 19% of cases. 35 So far, the available ECVs are diagnostic tools. However, prototypes already exist that allow the physician to take biopsies and to release hemostatic materials. 36 The characteristics of the available ECVs are summarized in Table 2.
Complications
The main complication is retention of the ECV which occurs in 0.75% to 5.8% of patients. 37 It can occur in up to 13% of patients with Crohn’s disease due to stenoses. 38 Symptoms of obstruction should be investigated with a “patient capsule” in a preliminary manner. Similarly, absence of stenosis should be documented with this device. It is a device composed of lactose and barium that is designed to dissolve 30 hours after ingestion. After this time only the radiopaque recording device remains. It can be located, but its small residual size of 3 × 13 mm allows it to transit even in stenotic areas. 39
Other less frequent complications are tracheobronchial aspiration, especially in elderly people with swallowing disorders. Very occasional cases of perforation have also been reported. 40
Analysis of findings requires time and concentration on the part of the examiner. Experienced gastroenterologists take approximately one hour to view the 50,000 images. 41 Software has been developed to decrease exam time. The first program designed to optimize interpretation of findings was the “red indicator” which is a system that identifies sites with increased red-scale pixels. Its objective is to facilitate detection of bleeding. 42 Nevertheless, use of this system still results in high rates of false positives and negatives. Therefore, the recommendation is that this tool should be considered simply for support and should not be totally trusted since its negative predictive value is less than 100%. 43
QuickView software also allows you to modify viewing speed and allows selection of images based on color. It can also be used to create short videos.
Although progress has been made in reading time and greater detection of injuries, the false negative rate still remains around 12%. 44. The latest generations of EVC such as CapsoCam have 360 ° fields of vision and record 5 photos per second. With a diagnostic performance similar to Pillcam (84.8% versus 81.8%), the CapsoCam requires longer reading time (32.0 minutes versus 26.2 minutes), but detects more lesions (108 lesions compared to 85 lesions). 45
Methods to overcome diagnostic difficulty in the duodenum are being designed based on directing a modified PillCam by means of an external magnet. 46 Other devices which can obtain biopsies and which have therapeutic options such as drug release and mechanical devices are also under development. 47 Examples of these are the NEMO (Nano-based capsule-Endoscopy with Molecular Imaging and Optical biopsy) and the VECTOR (versatile endoscopic capsule for recognition and treatment of gastrointestinal tumors). 48) There are already prototypes that have needles and nitinol clips inside a EVC to allow injections to be administered and clips placed. These could replace traditional invasive procedures that have high morbidity rates. 49 An important limitation of these models is their small size which implies using only small volumes of pre-loaded medicines and which cannot contain more than one mechanical device for hemostasis. 50
Another therapeutic possibility lies in production of local hemostasis with methods based on the generation of heat using an ECV preloaded with calcium oxide that can be released at the site of interest. 51 Similarly, insufflating devices that produce mechanical compression and others that expand upon contact with gastrointestinal fluids have been studied. 52
Push Enteroscopy
Push enteroscopy has been used since 1980 to evaluate the duodenum 50 to 100 cm distal of the ligament of Treitz by using “push and pull” to advance. Yield ranges from 3% to 70%, especially for vascular lesions, 53 but most lesions diagnosed have been found in accessible places through upper digestive endoscopy. The main disadvantage is the difficulty of advancing through loops, but it is useful because it allows treatment of proximal lesions. 54
Deep Enteroscopy (DE)
Deep enteroscopy aims to examine a greater length of the small intestine as a diagnostic and therapeutic method for stenoses (dilations), endoscopic hemostasis (argon plasma, clips, injections) and polypectomies. It is balloon-assisted with one or two balloons, uses spiral enteroscopy, and can be either antegrade (introduced through the mouth) or retrograde (anal). 55
Single Balloon Enteroscopy (SBE)
Equipment for this procedure was introduced in 2007. It has a single balloon at the distal end of the overtube. Via the antegrade route it reaches a depth from 133 cm to 256 cm beyond the ligament of Treitz and, by the retrograde route it reaches to 73 cm to 163 cm above the ileocecal valve. 56 Its diagnostic performance varies from 47% to 74%. The rate of adverse events is 1%. 57
Double Balloon Enteroscopy (DBE)
Double balloon enteroscopy was introduced in 2001. It uses an over-tube and a pump system with two inflatable balloons at its ends. These balloons allow the bowel to be folded through a series of advance and withdrawal cycles. 58 It must be performed with the patient under anesthesia and normally takes one to two hours. The depth of the intubation by the antegrade route is 240 to 360 cm beyond than the ligament of Treitz and from 102 to 140 cm proximal to the ileocecal valve by the retrograde route. 59 Its diagnostic performance is 60% to 80%, and its therapeutic yield is 40% to 73%. Examination of the entire small intestine is achieved in 16% to 86% of cases. 60 It has been shown to be a useful procedure for acute bleeding and has a low rate of recurrence of bleeding. 61 Minor complications occur in 9.1% of patients and include abdominal distension or pain, odynophagia and nausea. Major complications occur in 0.72% to 1% of patients and include acute pancreatitis, ileus, bleeding, aspiration pneumonia, and perforations. 62 Perforations usually occur during large polypectomies. Mortality is rare, with a rate of 0.05%. 63
Spiral Enteroscopy (SE)
Equipment for spiral enteroscopy includes a spiral overtube that advances into the small intestine through rotations. It can reach a depth of 176 cm to 250 cm. 64 When used alone, it has a diagnostic yield of 33%. 65 When used after positive capsule endoscopy, the yield is 57%. 66 Its main advantage is the short duration of the exam. Nevertheless, the retrograde approach is more difficult than it is for other methods. 67 Adverse effects vary: odynophagia has been reported in 12% of patients, mucosal tears in 27%, esophageal trauma in 7% and perforations in 0.3%. 2,68
Intraoperative Enteroscopy (IE)
Either laparoscopy or laparotomy is required to aid the advance of the enteroscope through the small intestine in this technique. The enteroscope can be introduced orally, rectally or through enterotomy which is generally recommended. 69 The diagnostic yield is between 58% and 88%. 70 Following a positive EVC finding, the yield is 87%. 71 The overall rebleeding rate is 13% to 60% after 19 months of follow-up. Complications occur in 0% to 52% of cases and include avulsion of the mesenteric vessels, prolonged ileus, hematomas, infections, and perforations. 72 Overall morbidity is 17%, mortality is 5%, and most of these cases are medical (11%) and surgical (22%) comorbidities. 73 This is an invasive method that should be reserved only for patients who have had recurrent bleeding and require multiple transfusions or hospitalizations after a broad negative evaluation or for whom enteroscopy cannot be performed due to stenosis or adhesions. 74
NaviAid AB (advance balloon)
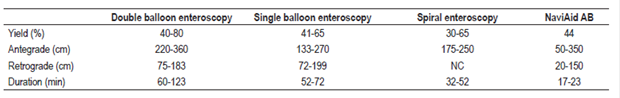
This method uses a standard colonoscope with a working channel of at least 3.7 mm through which a catheter with a balloon at the tip is advanced. The technique consists of advancing the catheter 30 to 40 cm distal to the tip of the colonoscope, insufflating the balloon, anchoring it in the intestinal loops and advancing the colonoscope along the catheter. 75 The cycle repeats itself and progresses quickly and securely through the small intestine. The catheter used has a length of 3.5 m, and the diameter of the balloon is 40 mm. 76 The device can be removed and replaced at the operator’s discretion, depending on whether it is necessary to take biopsies or perform therapeutic interventions. In preliminary studies, it has had a diagnostic yield of 44%, 77 even though it operates at shallower depth than conventional enteroscopy techniques. By the antegrade route, the depth of the insertion is 158 cm beyond the pylorus, and by the retrograde route the depth of insertion is 89 cm proximal to the ileocecal valve. The average time required to reach these distances is 15.5 minutes. 78 Table 3 summarizes characteristics of equipment used to examine the small intestine.
Radiographic techniques
Radiographic techniques include CT enterography, CT enteroclysis and MR enterography. Each of them has an average diagnostic yield of 45%. 79 CT enterography uses oral and venous contrast to detect inflammatory lesions, neoplasms and vascular abnormalities and has a diagnostic performance similar to that of ECV. 80 It can also be used to determine whether the antegrade or retrograde approach is most appropriate for enteroscopy. It is mainly useful for diagnosis of tumors for which it is superior to ECV with a diagnostic yield of 94.1% compared to 35.3%. Patient age of less than 40 years and severe bleeding are independent predictors of high diagnostic yield of CT enterography. 81. MR enterography is another option for patients with contraindications for ECV. Evidence for its use in cases of SBB is limited, 82 but it is indicated in those with contraindications to CT enterography and is preferred in young people because there is less radiation exposure than with CT enterography. 83 These imaging methods can be considered before ECV in patients with inflammatory bowel disease, undergoing radiation therapy, prior to small bowel surgery and when there is suspicion of stenosis in the small intestine. 84
Computed Tomography Angiography
Computed tomography angiography is an option for patients who are intolerant to oral contrast. It has been shown to have the capacity of detecting slow bleeding rates of 0.3 mL/min. 85
Technetium-99m-Labeled Red Blood Cell Scans
Technetium-99m-labeled red blood cell scans can detect gastrointestinal bleeding at a rate of 0.1-0.4 mL/min prior to selective angiography, but studies have shown its accuracy for locating the source of bleeding to be poor. 86
Tc-99m Pertechnetate Scans
Tc-99m pertechnetate scans are useful for diagnosing Meckel’s diverticulum. This technique is based on uptake of pertechnetate anions by the ectopic gastric mucosa. It has a sensitivity of 64% to 100%, 87 but false positive results may occur in the following situations: arteriovenous malformations, inflammatory lesions, ulcers, obstructions, intussusception, duplication cysts and ectopic gastric mucosa. 88.
Angiography
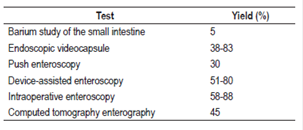
The objective of angiography is topographic localization and therapeutic embolization. 89 Blood loss must be at least 0.5-1 mL/min for angiography to detect the site of bleeding, 90 and this determines its diagnostic performance which is between 50% and 75% for active bleeding and less than 50% for slow or limited bleeding. 91 Potential complications include pseudoaneurysms, arterial thrombosis, renal failure, dissection and intestinal infarction. There may also be infections or bleeding at the catheter site which has an incidence of 10% of cases. 92 Table 4 summarizes the diagnostic performance rates of techniques to evaluate the small intestine.
Diagnostic approach
During the initial approach, the diagnostic yield of ECV and enteroscopy for overt bleeding is 92%, but it is only 44% for occult bleeding. 93. The probability of making the diagnosis with EVC or enteroscopy decreases as time passes. If manifest bleeding is studied within 10 to 14 days of the onset of symptoms, it is possible to identify the lesion in 67% of cases, but after three to four weeks it is reduced to 33%. 94 EVC is the exam of choice for study of possible SBB since management strategy may change in 33% to 66% of patients and since this technique has been shown to be superior to push enteroscopy (63% versus to 23%). 95 ECV has also reduced the number of hospitalizations, additional studies and the need for blood transfusions. Its diagnostic yield (73% -93%) and therapeutic yield (57% -73%) are higher than those for enteroscopy. 96 Due to its high negative predictive value, enteroscopy can be avoided in patients with a low probability of positive findings in the small intestine prior to the examination. If the results are negative and the patient’s clinical status is stable, follow-up can be done without additional tests given the low rebleeding rate. 97 However, for patients with negative results who are taking anticoagulants, close observation is required and other modalities should be considered as alternatives, but there are still no clear indications about which technique to use or at what time a procedure is appropriate.
Repetition of ECV is recommended for patients with hemoglobin decreases of 4 g/dL or more and for patients whose bleeding has become manifest. In these cases, repetition has been reported to increase diagnostic performance. 98 Another option is to perform double balloon enteroscopy which can detect the source of the hemorrhaging in 30% of this group of patients. Selection of the type of deep enteroscopy depends on availability and expertise in the management of the technique. In a prospective multicenter trial that compared SBE and DBE, DBE had a total enteroscopy rate three times greater than that of SBE (66% vs. 22%, p <0.0001), but the therapeutic yield was not statistically significant due to the small number of participants (72% versus 48%, p <0.025). 99 One review has compared the diagnostic performance of single balloon, double balloon, and spiral enteroscopy and found similar results (53.9%, 64.4%, and 47%, respectively). Regarding the duration of the procedure, the fastest procedure was spiral enteroscopy (Oral: 41.0 min., Anal: 46 min.) followed by SBE (Oral: 59.8 min., Anal: 68.8 min.) and DBE (Oral: 71.6 min., Anal: 84.5 min.).
Therapeutic interventions had better results with DBE (40.1%) than with SBE (26.8%) and spiral enteroscopy (29.7%). 100 A difference of insertion times has been reported between DBE and spiral enteroscopy: spiral enteroscopy takes about 43 minutes while DBE takes about 65 minutes ( p = 0.007), but DBE has a much longer reach length (310 cm versus at 250 cm, p = 0.004). The average procedure time for the antegrade approach has been estimated at 79 ± 15 minutes for DBE, 65 ± 16 minutes for SBE, and 35 ± 6 minutes for spiral enteroscopy. 101 Metaanalyses that have compared these tests for the study of the small intestine have had varying results, with limitations due to heterogeneity and wide confidence intervals, which decrease the levels of evidence. 102
Massive bleeding
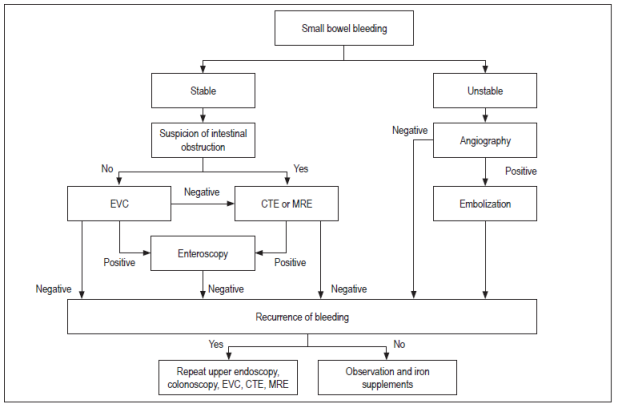
For hemodynamically unstable patients, conventional angiography or deep enteroscopy may be considered for urgent care because of the possibility of therapeutic intervention. If the patient has active bleeding but is hemodynamically stable, CT enterography or CT angiography can be performed to identify the site of bleeding and then guide treatment. 103 For young patients with overt hemorrhaging a Tc-99m pertechnetate scan should be performed to rule out Meckel’s diverticulum. In patients with manifest gastrointestinal bleeding and slower bleeding rates (0.1-0.2 mL/min), a scan with marked red blood cells should be performed if deep enteroscopy or ECV cannot be performed. 104 Figure 2 outlines the approach to small bowel bleeding.
Clinical evolution and rebleeding
The rebleeding rate varies in different publications. This inconsistency is related to the institution, the duration of the follow-up and the cause of the hemorrhage. Independent risk factors for rebleeding include multiple transfusions and comorbidities such as chronic renal failure, use of anticoagulation and diabetes mellitus. 105 The rate of rebleeding after intervention to treat lesions detected by ECV has been studied and found to be 50% in patients with angiodysplasia despite endoscopic intervention. Similarly, it is greater for patients with lesions without clinical relevance regardless of whether an endoscopic procedure was performed. 106 In interventions performed as the result of ECV findings, between 50% and 66% of patients require no transfusions and experience no recurrence of bleeding. 84,85 In other words, the risk of rebleeding after a negative ECV is very low, between 5.6% and 11%. 107
Within the 12 months following deep enteroscopy, recurrences of overt bleeding occurred in 34% of patients, compared with 13% of patients with occult bleeding (p = 0.06) 87. These recurrence rates, however, were not significant at 30 months of follow-up (27% vs. 20%) 87. With negative DBE, the rate is 30% to 40% 42 and, with negative EE, 26% after 2 years of follow-up 108.
Conclusions
Recently, gastrointestinal hemorrhages have been redefined and a new term “small bowel bleeding” (SBB) has been introduced. SBB explains a high percentage of those lesions previously classified as “of obscure origin”. This is in large part due to the possibility of detecting lesions in previously inaccessible sites which has modified the clinical and therapeutic approach. The endoscopic videocapsule continues to be the main diagnostic tool. Prototypes now being developed will offer therapeutic options in addition to the current role of guiding the approach to interventions. Enteroscopy techniques offer similar diagnostic performances, but they must be chosen according to the availability and experience of the medical center. Among radiological techniques, CT enterography is preferred because of its characteristics, but there are multiple possibilities if there is any contraindication. Angiography is the first choice for clinically unstable patients since it offers rapid therapeutic intervention. There is no doubt about the great importance of this topic and the interest it has aroused worldwide with exponential development of various techniques that increase diagnostic yield and allow treatments that result in lower rates of related morbidity.
Acknowledgements
None declared by the authors.
REFERENCES
1. Pasha SF, Hara AK, Leighton JA. Diagnostic evaluation and management of obscure gastrointestinal bleeding: a changing paradigm. Gastroenterol Hepatol. 2009;5(12):839-50. [ Links ]
2. Gerson LB, Fidler JL, Cave DR, et al. ACG Clinical guideline: diagnosis and management of small bowel bleeding. Am J Gastroenterol 2015; 110(9):1265-87. Doi: https://doi.org/10.1038/ajg.2015.246 [ Links ]
3. Ohmiya N , Nakagawa Y, Nagasaka M, et al . Obscure gastrointestinal bleeding: diagnosis and treatment. Dig Endosc. 2015;27(3):285-94. Doi: https://doi.org/10.1111/den.12423 [ Links ]
4. Dulic-Lakovic E, Dulic M, Hubner D, et al. Bleeding Dieulafoy lesions of the small bowel: a systematic study on the epidemiology and efficacy of enteroscopic treatment. Gastrointest Endosc. 2011;74(3):573-580. Doi: https://doi.org/10.1016/j.gie.2011.05.027 [ Links ]
5. Min Naut ER. The approach to occult gastrointestinal bleed. Med Clin North Am. 2016;100(5):1047-56. Doi: https://doi.org/10.1016/j.mcna.2016.04.013 [ Links ]
6. Kaufman D, Leslie G, Marya N, et al. Small Intestinal angioectasia: characterization, risk factors, and rebleeding. J Clin Gastroenterol. 2016. Doi: https://doi.org/10.1097/MCG.0000000000000663 [ Links ]
7. Limsrivilai J, Srisajjakul S, Pongprasobchai S, et al. A prospective blinded comparison of video capsule endoscopy versus computed tomography enterography in potential small bowel bleeding: clinical utility of computed tomography enterography. J Clin Gastroenterol. 2016. [ Links ]
8. Yatagai N, Ueyama H, Shibuya T, et al. Obscure gastrointestinal bleeding caused by small intestinal lipoma: a case report J Med Case Rep. 2016;10(1):226. [ Links ]
9. Liu K, Kaffes AJ. Review article: the diagnosis and investigation of obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2011;34(4):416-23. Doi: https://doi.org/10.1111/j.1365-2036.2011.04744.x [ Links ]
10. Rondonotti E, Pennazio M, Toth E, et al. Small bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40(6):488-95. Doi: https://doi.org/10.1055/s-2007-995783 [ Links ]
11. Pasha SF, Sharma VK, Carey EJ, et al. Utility of video capsule endoscopy in the detection of small bowel tumors. A single center experience of 1000 consecutive patients. Proceedings of the 6th International Conference on Capsule Endoscopy. June 8-10; Madrid, Spain, 2007. Nueva York: McGraw-Hill; 2007. p. 45. [ Links ]
12. Goenka M.K, Majumder S, Goenka U. Capsule endoscopy: present status and future expectation. World J Gastroenterol 2014;20(29):10024-37. Doi: https://doi.org/10.3748/wjg.v20.i29.10024 [ Links ]
13. Santhakumar C, Liu K. Evaluation and outcomes of patients with obscure gastrointestinal bleeding. World J Gastrointest Pathophysiol. 2014;5(4):479-86. Doi: https://doi.org/10.4291/wjgp.v5.i4.479 [ Links ]
14. Upchurch BR, Vargo JJ. Small bowel enteroscopy. Rev Gastroenterol Disord. 2008;8(3):169-77. [ Links ]
15. Lewis BS. Small intestinal bleeding. Gastroenterol Clin North Am. 2000;29(1):67-95. Doi: https://doi.org/10.1016/S0889-8553(05)70108-4 [ Links ]
16. Singh A, Baptista V, Stoicov C, et al. Evaluation of small bowel bleeding. Curr Opin Gastroenterol. 2013;29(2):119-24. Doi: https://doi.org/10.1097/MOG.0b013e32835bdc1a [ Links ]
17. Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47(4):352-76. Doi: https://doi.org/10.1055/s-0034-1391855 [ Links ]
18. Rondonotti E, Villa F, Mulder CJ, et al. Small bowel capsule endoscopy in 2007: indications, risks and limitations. World J Gastroenterol. 2007;13(46):6140-9. Doi: https://doi.org/10.3748/wjg.13.6140 [ Links ]
19. Mishkin DS, Chuttani R, Croffie J, et al. ASGE Technology Status Evaluation Report: wireless capsule endoscopy. Gastrointest Endosc. 2006;63(4):539-45. Doi: https://doi.org/10.1016/j.gie.2006.01.014 [ Links ]
20. Carey EJ, Leighton JA, Heigh RI, et al. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102(1):89-95. Doi: https://doi.org/10.1111/j.1572-0241.2006.00941.x [ Links ]
21. Singh A, Marshall C, Chaudhuri B, et al. Timing of video capsule endoscopy relative to overt obscure GI bleeding: implications from a retrospective study. Gastrointest Endosc. 2013;77(5):761-6. Doi: https://doi.org/10.1016/j.gie.2012.11.041 [ Links ]
22. Yamada A, Watabe H, Kobayashi Y, et al. Timing of capsule endoscopy influences the diagnosis and outcome in obscure-overt gastrointestinal bleeding. Hepatogastroenterology. 2012;59(115):676-9. [ Links ]
23. Rocchi A, Benchimol EI, Bernstein CN, et al. Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26(11):811-7. Doi: https://doi.org/10.1155/2012/984575 [ Links ]
24. Lai LH, Wong GL, Chow DK, et al. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006;101(6):1224-8. Doi: https://doi.org/10.1111/j.1572-0241.2006.00565.x [ Links ]
25. Savale L, Soussan B, Ramírez S, et al. Outcome of patients with obscure gastrointestinal bleeding after negative capsule endoscopy: results of a one-year follow-up study. Gastroenterol Clin Biol. 2010;34(11):606-11. Doi: https://doi.org/10.1016/j.gcb.2010.06.009 [ Links ]
26. Iwamoto J, Mizokami Y, Shimokobe K, et al. The clinical outcome of capsule endoscopy in patients with obscure gastrointestinal bleeding. Hepatogastroenterology. 2011;58(106):301-5. [ Links ]
27. Koh SJ, Im JP, Kim JW, et al. Long-term outcome in patients with obscure gastrointestinal bleeding after negative capsule endoscopy. World J Gastroenterol. 2013;19(10):1632-8. Doi: https://doi.org/10.3748/wjg.v19.i10.1632 [ Links ]
28. Kim JB, Ye BD, Song Y, et al. Frequency of rebleeding events in obscure gastrointestinal bleeding with negative capsule endoscopy. J Gastroenterol Hepatol. 2013;28(5):834-40. Doi: https://doi.org/10.1111/jgh.12145 [ Links ]
29. Keller J, Fibbe C, Volke F, et al. Inspection of the human stomach using remote-controlled capsule endoscopy: a feasibility study in healthy volunteers (with videos). Gastrointest Endosc. 2011;73:22-8. Doi: https://doi.org/10.1016/j.gie.2010.08.053 [ Links ]
30. Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole and placebo. Clin Gastroenterol Hepatol. 2005;3(2):133-41. Doi: https://doi.org/10.1016/S1542-3565(04)00619-6 [ Links ]
31. Lewis BS, Eisen GM, Friedman S. A pooled analysis to evaluate results of capsule endoscopy trials. Endoscopy. 2005;37(10):960-5. Doi: https://doi.org/10.1055/s-2005-870353 [ Links ]
32. Appleyard M, Fireman Z, Glukhovsky A, et al. A randomized trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small-bowel lesions. Gastroenterology. 2000;119(6):1431-8. Doi: https://doi.org/10.1053/gast.2000.20844 [ Links ]
33. Clarke JO, Giday SA, Magno P, et al. How good is capsule endoscopy for detection of periampullary lesions? Results of a tertiary-referral center. Gastrointest Endosc. 2008;68(2):267-72. Doi: https://doi.org/10.1016/j.gie.2007.11.055 [ Links ]
34. Pennazio M, Rondonotti E, de Franchis R. Capsule endoscopy in neoplastic diseases. World J Gastroenterol. 2008;14(34):5245-53. Doi: https://doi.org/10.3748/wjg.14.5245 [ Links ]
35. Nadler M, Eliakim R. The role of capsule endoscopy in acute gastrointestinal bleeding. Therap Adv Gastroenterol. 2014;7:87-92. Doi: https://doi.org/10.1177/1756283X13504727 [ Links ]
36. Van de Bruaene C, De Looze D, Hindryckx P. Small bowel capsule endoscopy: where are we after almost 15 years of use? World J Gastrointest Endosc. 2015;7:13-36. Doi: https://doi.org/10.4253/wjge.v7.i1.13 [ Links ]
37. Koulaouzidis A, Karargyris A, et al. Utility of 3-dimensional image reconstruction in the diagnosis of small-bowel masses in capsule endoscopy (with video). Gastrointest Endosc. 2014;80(4):642-51. Doi: https://doi.org/10.1016/j.gie.2014.04.057 [ Links ]
38. Pioche M, Vanbiervliet G, Jacob P, et al. Prospective randomized comparison between axial- and lateral-viewing capsule endoscopy systems in patients with obscure digestive bleeding. Endoscopy. 2014;46:479-84. [ Links ]
39. Swain P, Toor A, Volke F, et al. Remote magnetic manipulation of a wireless capsule endoscope in the esophagus and stomach of humans (with videos). Gastrointest Endosc. 2010;71(7):1290-3. Doi: https://doi.org/10.1016/j.gie.2010.01.064 [ Links ]
40. Rey JF, Ogata H, Hosoe N, et al. Blinded nonrandomized comparative study of gastric examination with a magnetically guided capsule endoscope and standard videoendoscope. Gastrointest Endosc. 2012;75(2):373-81. Doi: https://doi.org/10.1016/j.gie.2011.09.030 [ Links ]
41. Ortora G, Valdastri P, Susilo E, et al. Propeller-based wireless device for active capsular endoscopy in the gastric district. Minim Invasive Ther Allied Technol. 2009;18(5):280-90. Doi: https://doi.org/10.1080/13645700903201167 [ Links ]
42. Yim S, Gultepe E, Gracias DH, et al. Biopsy using a magnetic capsule endoscope carrying, releasing, and retrieving untethered microgrippers. IEEE Trans Biomed Eng. 2014;61(2):513-21. Doi: https://doi.org/10.1109/TBME.2013.2283369 [ Links ]
43. Muñoz F, Alici G, Li W. A review of drug delivery systems for capsule endoscopy. Adv Drug Deliv Rev. 2014;71:77-85. Doi: https://doi.org/10.1016/j.addr.2013.12.007 [ Links ]
44. Ryan Scott. Advances in capsule endoscopy gastroenterology & hepatology. Gastroenterol Hepatol. 2015;11:612-27. [ Links ]
45. Ross A, Mehdizadeh S, Tokar J, et al. Double balloon enteroscopy detects small bowel mass lesions missed by capsule endoscopy. Dig Dis Sci. 2008;53(8):2140-3. Doi: https://doi.org/10.1007/s10620-007-0110-0 [ Links ]
46. Schostek S, Schurr MO. European research on wireless endoscopy the VECTOR project. Stud Health Technol Inform. 2013;189:193-9. [ Links ]
47. Hale MF, Sidhu R, McAlindon ME. Capsule endoscopy: current practice and future directions. World J Gastroenterol. 2014;20(24):7752-9. Doi: https://doi.org/10.3748/wjg.v20.i24.7752 [ Links ]
48. Valdastri P, Quaglia C, Susilo E, et al. Wireless therapeutic endoscopic capsule: in vivo experiment. Endoscopy. 2008;40(12):979-82. Doi: https://doi.org/10.1055/s-0028-1103424 [ Links ]
49. Pennazio M. Capsule endoscopy: where are we after 6 years of clinical use? Dig Liver Dis 2006;38(12):867-78. Doi: https://doi.org/10.1016/j.dld.2006.09.007 [ Links ]
50. Fry LC, De Petris G, Swain JM, et al. Impaction and fracture of a video capsule in the small bowel requiring laparotomy for removal of the capsule fragments. Endoscopy. 2005;37(7):674-6. Doi: https://doi.org/10.1055/s-2005-870252 [ Links ]
51. Cheifetz AS, Kornbluth AA, Legnani P, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol. 2006;101(10):2218-22. Doi: https://doi.org/10.1111/j.1572-0241.2006.00761.x [ Links ]
52. Health Quality Ontario. Capsule endoscopy in the assessment of obscure gastrointestinal bleeding: an evidence-based analysis. Ont Health Technol Assess Ser. 2015;15(1):1-55. [ Links ]
53. Bhattarai M, Bansal P, Khan Y. Longest duration of retention of video capsule: a case report and literature review. World J Gastrointest Endosc. 2013;5(7):352-5. Doi: https://doi.org/10.4253/wjge.v5.i7.352 [ Links ]
54. Repici A, Barbon V, De Angelis C, et al. Acute small-bowel perforation secondary to capsule endoscopy. Gastrointest Endosc. 2008;67(1):180-3. Doi: https://doi.org/10.1016/j.gie.2007.05.044 [ Links ]
55. Lin S, Branch MS, Shetzline M. The importance of indication in the diagnostic value of push enteroscopy. Endoscopy. 2003;35(4):315-21. Doi: https://doi.org/10.1055/s-2003-38144 [ Links ]
56. Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133(5):1697-717. Doi: https://doi.org/10.1053/j.gastro.2007.06.008 / https://doi.org/10.1053/j.gastro.2007.06.007 [ Links ]
57. Linder J, Cheruvattath R, Truss C, et al. Diagnostic yield and clinical implications of push enteroscopy: results from a nonspecialized center. J Clin Gastroenterol. 2002;35(5):383-6. Doi: https://doi.org/10.1097/00004836-200211000-00005 [ Links ]
58. Sidhu R, McAlindon ME, Kapur K, et al. Push enteroscopy in the era of capsule endoscopy. J Clin Gastroenterol. 2008;42(1):54-8. Doi: https://doi.org/10.1097/01.mcg.0000225655.85060.74 [ Links ]
59. Sidhu R, Sanders DS. Double-balloon enteroscopy in the elderly with obscure gastrointestinal bleeding: safety and feasibility. Eur J Gastroenterol Hepatol. 2013;25(10):1230-4. Doi: https://doi.org/10.1097/MEG.0b013e3283630f1b [ Links ]
60. Baptista V, Marya N, Singh A, et al. Continuing challenges in the diagnosis and management of obscure gastrointestinal bleeding. World J Gastrointest Pathophysiol. 2014;5(4):523-33. Doi: https://doi.org/10.4291/wjgp.v5.i4.523 [ Links ]
61. Mönkemüller K, Neumann H, Meyer F, et al. A retrospective analysis of emergency double-balloon enteroscopy for small-bowel bleeding. Endoscopy. 2009;41(8):715-7. Doi: https://doi.org/10.1055/s-0029-1214974 [ Links ]
62. Aniwan S, Viriyautsahakul V, Rerknimitr R, et al. Urgent double balloon endoscopy provides higher yields than non-urgent double balloon endoscopy in overt obscure gastrointestinal bleeding. Endosc Int Open. 2014;2(2):E90-5. Doi: https://doi.org/10.1055/s-0034-1365543 [ Links ]
63. Xin L, Liao Z, Jiang YP, et al. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc. 2011;74(3):563-70.Doi:https://doi.org/10.1016/j.gie.2011.03.1239 [ Links ]
64. Moschler O, May AD, Muller MK, et al. Complications in double-balloon enteroscopy: results of the German DBE register. Z Gastroenterol. 2008;46(3):266-70. [ Links ]
65. Khashab MA, Pasha SF, Muthusamy VR, et al. ASGE: the role of deep enteroscopy in the management of small-bowel Disorders. Gastrointest Endosc. 2015;82(4):600-7. Doi: https://doi.org/10.1016/j.gie.2015.06.046 [ Links ]
66. Buscaglia JM, Richards R, Wilkinson MN, et al. Diagnostic yield of spiral enteroscopy when performed for the evaluation of abnormal capsule endoscopy findings. J Clin Gastroenterol. 2011;45(4):342-6. Doi: https://doi.org/10.1097/MCG.0b013e3181eeb74b [ Links ]
67. Schembre DB, Ross AS. Spiral enteroscopy: a new twist on over tuve assisted endoscopy. Gastrointest Endosc. 2009;69(2):333-6. Doi: https://doi.org/10.1016/j.gie.2008.09.011 [ Links ]
68. Douard R, Wind P, Berger A, et al. Role of intraoperative enteroscopy in the management of obscure gastointestinal bleeding at the time of video-capsule endoscopy. Am J Surg. 2009;198(1):6-11. Doi: https://doi.org/10.1016/j.amjsurg.2008.06.036 [ Links ]
69. Bonnet S, Douard R, Malamut G, et al. Intraoperative enteroscopy in the management of obscure gastrointestinal bleeding. Dig Liver Dis. 2013;45(4):277-84. [ Links ]
70. Murino A, Vlachou E, Fraser C, et al. Deep enteroscopy using a conventional colonoscope and through-the-scope balloon catheter system: How deep is deep? Gastrointest Endosc. 2016;84(5):882-3. Doi: https://doi.org/10.1016/j.gie.2016.01.031 [ Links ]
71. Neumann H. Through-the-scope deep enteroscopy (TTS-DE): new technique for deep small-bowel endoscopy - a pilot study. Z Gastroenterol. 2013;51:K259. Doi: https://doi.org/10.1055/s-0033-1352899 [ Links ]
72. Deep enteroscopy with a conventional colonoscope: initial multicenter study by using a through-the-scope balloon catheter system. Gastrointest Endosc. 2015. [ Links ]
73. Howarth DM. The role of nuclear medicine in the detection of acute gastrointestinal bleeding. Semin Nucl Med. 2006;36(2):133-46. Doi: https://doi.org/10.1053/j.semnuclmed.2005.11.001 [ Links ]
74. Lee SS, Oh TS, Kim HJ, et al. Obscure gastrointestinal bleeding: diagnostic performance of multidetector CT enterography. Radiology. 2011;259(3):739-48. Doi: https://doi.org/10.1148/radiol.11101936 [ Links ]
75. Filippone A, Cianci R, Milano A, et al. Obscure gastrointestinal bleeding and small bowel pathology: comparison between wireless capsule endoscopy and multidetector-row CT enteroclysis. Abdom Imaging. 2008;33(4):398-406. Doi: https://doi.org/10.1007/s00261-007-9271-8 [ Links ]
76. Heo HM, Park CH, Lim JS, et al. The role of capsule endoscopy after negative CT enterography in patients with obscure gastrointestinal bleeding. Eur Radiol. 2012;22(6):1159-66. Doi: https://doi.org/10.1007/s00330-011-2374-1 [ Links ]
77. Browder W, Cerise EJ, Litwin MS. Impact of emergency angiography in massive lower gastrointestinal bleeding. Ann Surg. 1986;204(5):530-6. Doi: https://doi.org/10.1097/00000658-198611000-00004 [ Links ]
78. Leung WK, Ho SS, Suen BY, et al. Capsule endoscopy or angiography in patients with acute overt obscure gastrointestinal bleeding: a prospective randomized study with long-term follow-up. Am J Gastroenterol. 2012;107(9):1370-6. Doi: https://doi.org/10.1038/ajg.2012.212 [ Links ]
79. Rahn NH 3rd, Tishler JM, Han SY, et al. Diagnostic and interventional angiography in acute gastrointestinal hemorrhage. Radiology. 1982;143(2):361-6. Doi: https://doi.org/10.1148/radiology.143.2.6978500 [ Links ]
80. Silverstein FE, Gilbert DA, Tedesco FJ, et al. The national ASGE survey on upper gastrointestinal bleeding. I. Study design and baseline data. Gastrointest Endosc. 1981;27(2):73-9. Doi: https://doi.org/10.1016/S0016-5107(81)73155-9 [ Links ]
81. Strate LL. Lower GI bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34(4):643-64. Doi: https://doi.org/10.1016/j.gtc.2005.08.007 [ Links ]
82. Leaper M, Johnston MJ, Barclay M, et al. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy. 2004;36(6):499-503. Doi: https://doi.org/10.1055/s-2004-814399 [ Links ]
83. Descamps C, Schmit A, Van Gossum A. “Missed” upper gastrointestinal tract lesions may explain “occult” bleeding. Endoscopy. 1999;31(6):452-5. Doi: https://doi.org/10.1055/s-1999-151 [ Links ]
84. Chak A, Koehler MK, Sundaram SN, et al. Diagnostic and therapeutic impact of push enteroscopy: analysis of factors associated with positive findings. Gastrointest Endosc. 1998;47(1):18-22. Doi: https://doi.org/10.1016/S0016-5107(98)70293-7 [ Links ]
85. Pennazio M, Santucci R, Rondonotti E, et al. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126(3):643-53. Doi: https://doi.org/10.1053/j.gastro.2003.11.057 [ Links ]
86. Arakawa D, Ohmiya N, Nakamura M, et al. Outcome after enteroscopy for patients with obscure GI bleeding: diagnostic comparison between double-balloon endoscopy and videocapsule endoscopy. Gastrointest Endosc. 2009;69(4):866-74. Doi: https://doi.org/10.1016/j.gie.2008.06.008 [ Links ]
87. Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100(11):2407-18. Doi: https://doi.org/10.1111/j.1572-0241.2005.00274.x [ Links ]
88. de Leusse A, Vahedi K, Edery J, et al. Capsule endoscopy or push enteroscopy for first-line exploration of obscure gastrointestinal bleeding? Gastroenterology. 2007;132(3):855-62. Doi: https://doi.org/10.1053/j.gastro.2006.12.002 [ Links ]
89. Kaffes AJ, Siah C, Koo JH. Clinical outcomes after doubleballoon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. 2007;66(2):304-9. Doi: https://doi.org/10.1016/j.gie.2007.02.044 [ Links ]
90. Fry LC, Neumann H, Jovanovic I, et al. Capsule endoscopy increases the diagnostic yield of double balloon enteroscopy in patients being investigated for obscure gastrointestinal bleeding. Arch Gastroenterohepatol. 2012;29(1):9-14. [ Links ]
91. Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006;38(1):49-58. Doi: https://doi.org/10.1055/s-2005-921176 [ Links ]
92. Hendel JW, Vilmann P, Jensen T. Double-balloon endoscopy: who needs it? Scand J Gastroenterol. 2008;43(3):363-7. Doi: https://doi.org/10.1080/00365520701799468 [ Links ]
93. Riccioni ME, Urgesi R, Cianci R, et al. Negative capsule endoscopy in patients with obscure gastrointestinal bleeding reliable: recurrence of bleeding on long-term follow-up. World J Gastroenterol. 2013;19(28):4520-5. Doi: https://doi.org/10.3748/wjg.v19.i28.4520 [ Links ]
94. Fisher L, Lee Krinsky M, Anderson MA, et al. The role of endoscopy in the management of obscure GI bleeding. Gastrointest Endosc. 2010;72(3):471-9. Doi: https://doi.org/10.1016/j.gie.2010.04.032 [ Links ]
95. Gerson LB. Small bowel endoscopy: cost-effectiveness of the different approaches. Best Pract Res Clin Gastroenterol. 2012;26(3):325-35. Doi: https://doi.org/10.1016/j.bpg.2012.01.018 [ Links ]
96. Tae CH, Shim KN. Should capsule endoscopy be the first test for every obscure gastrointestinal bleeding? Clin Endosc. 2014;47(5):409-14. Doi: https://doi.org/10.5946/ce.2014.47.5.409 [ Links ]
97. Viazis N, Papaxoinis K, Vlachogiannakos J, et al. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc. 2009;69(4):850-6. Doi: https://doi.org/10.1016/j.gie.2008.05.053 [ Links ]
98. Teshima CW, Kuipers EJ, van Zanten SV, et al. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011;26(5):796-801. Doi: https://doi.org/10.1111/j.1440-1746.2010.06530.x [ Links ]
99. May A, Färber M, Aschmoneit I, et al. Prospective multicenter trial comparing push-and-pull enteroscopy with the single- and double-balloon techniques in patients with small-bowel disorders. Am J Gastroenterol. 2010;105(3):575-81. Doi: https://doi.org/10.1038/ajg.2009.712 [ Links ]
100. Lenz P, Domagk D. Double- vs. single-balloon vs. spiral enteroscopy. Best Pract Res Clin Gastroenterol. 2012;26(3):303-13. Doi: https://doi.org/10.1016/j.bpg.2012.01.021 [ Links ]
101. Endo H, Matsuhashi N, Inamori M, et al. Rebleeding rate after interventional therapy directed by capsule endoscopy in patients with obscure gastrointestinal bleeding. BMC Gastroenterol. 2008;8:12-7. Doi: https://doi.org/10.1186/1471-230X-8-12 [ Links ]
102. Delvaux M, Fassler I, Gay G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy. 2004;36(12):1067-73. Doi: https://doi.org/10.1055/s-2004-826034 [ Links ]
103. Estévez E, González-Conde B, Vázquez-Iglesias JL, et al. Diagnostic yield and clinical outcomes after capsule endoscopy in 100 consecutive patients with obscure gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2006;18(18):881-8. Doi: https://doi.org/10.1097/00042737-200608000-00014 [ Links ]
104. Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc. 2008;68(6):1122-7. Doi: https://doi.org/10.1016/j.gie.2008.06.054 [ Links ]
105. Gerson LB, Batenic MA, Newsom SL, et al. Long-term outcomes after double-balloon enteroscopy for obscure gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2009;7(6):664-9. Doi: https://doi.org/10.1016/j.cgh.2009.01.021 [ Links ]
106. Fujita M, Manabe N, Honda K, et al. Long-term outcome after double-balloon endoscopy in patients with obscure gastrointestinal bleeding. Digestion. 2010;82(3):173-8. Doi: https://doi.org/10.1159/000313360 [ Links ]
107. Shinozaki S, Yano T, Sakamoto H, et al. Long-term outcomes in patients with overt obscure gastrointestinal bleeding after negative double-balloon endoscopy. Dig Dis Sci. 2015;60(12):3691-6. Doi: https://doi.org/10.1007/s10620-015-3792-8 [ Links ]
108. Williamson JB, Judah JR, Gaidos JK, et al. Prospective evaluation of the long-term outcomes after deep small-bowel spiral enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc. 2012;76(4):771-8. Doi: https://doi.org/10.1016/j.gie.2012.05.025 [ Links ]
Received: October 18, 2016; Accepted: July 08, 2017











 text in
text in