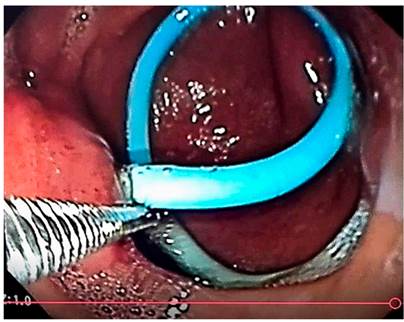
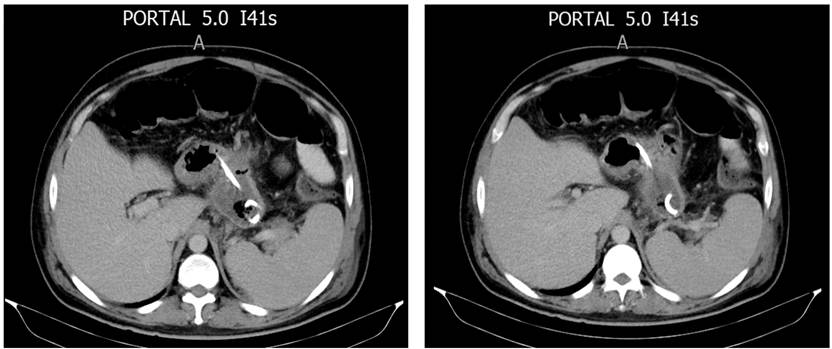
Introduction
Pancreatic duct obstruction (PDO) is defined as the partial or total destruction of the primary pancreatic duct, usually in the neck or body of the pancreas, secondary to pancreatic necrosis in severe pancreatitis. As the compromised segment continues with its exocrine function, intra- or peripancreatic collections, external pancreatic fistulas (EPF), or persistent discharge of pancreatic fluid through the surgical or percutaneous catheter occur.
In the case of total disruption, the possibility of recurrence of collections after endoscopic or percutaneous treatment is greater, and resection of the affected pancreatic segment is sometimes necessary as definitive treatment. Given the complexity of this pathology, different therapeutic options are proposed without having defined a gold standard until now1. PDO development is also related to traumatic surgical procedures such as endoscopic or surgical necrosectomy2,3, used to remove infected pancreatic necrosis (IPN).
The imaging diagnosis of PDO is made by endoscopic pancreatography, MRI, or tomography. After PDO, complications could be recurrent pancreatic fluid collections, ascites, fistula formation, pseudoaneurysm, diabetes, and exocrine insufficiency4,5.
This paper aims to describe and discuss the results of the percutaneous-endoscopic approach in a series of six patients with PDO.
Case presentation
A series of six patients treated between 2015 and 2018 diagnosed with IPN and later with PDO is presented. There is evidence that necrosis of the pancreas is associated with PDO development in approximately 20%-30% of cases1,3. All the patients included in this study exhibited PDO as a complication after the resolution of IPN.
The following criteria were considered to select patients: having undergone surgery for IPN by transgastric percutaneous drainage, having a diagnosis of total or partial PDO, being older than 15 years, having been discharged and monitored.
Regarding the characteristics of the patients, 5 were men with a mean age of 39 years-all patients presented with IPN as the initial indication for percutaneous drainage. The percutaneous approaches to treat IPN were imaging-guided transgastric, transperitoneal, and transretroperitoneal drainage, combining them depending on each clinical case (Figures 1 and 2, Table 1).
Table 1 Characteristics of patients with IPN
| Patient | Intervention time from the onset of pancreatitis (day) | Percutaneous procedures (n) | Percutaneous approaches used (n) | Isolated microorganisms |
|---|---|---|---|---|
| 1 | 38 | 8 | Transgastric (4) Retroperitoneal (1) Transperitoneal (3) | Escherichia coli, Pseudomonas aeruginosa, Enterobacter cloacae, Proteus mirabilis |
| 2 | 20 | 2 | Transgastric (1) Retroperitoneal (1) | Citobacter freundii |
| 3 | 18 | 2 | Transgastric (2) | Acinetobacter baumanii |
| 4 | 22 | 2 | Transgastric (2) | Enterobacter aerogenes |
| 5 | 25 | 4 | Transperitoneal (2) Transgastric (2) | Candida krusei, E. aerogenes, Proteus mirabilis |
| 6 | 50 | 2 | Transgastric (2) | Staphylococcus aureus |
Multipurpose pigtail catheters from 10 to 12 Fr were used. The average resolution time of the IPN was 42 days.
Regarding PDO diagnosis, four were by computerized axial tomography (CAT) and two by magnetic resonance imaging (MRI). As a result, four (66.6%) partial and two (33.4%) total PDOs were obtained. All PDOs were in the body of the pancreas.
Because PDO did not resolve with medical treatment with octreotide and total parenteral nutrition over 14 days, different prostheses were placed using the transgastric catheter path. The prostheses used were: two double J biliary stents (33.4%), one double J catheter (16.6%), one inverted pigtail catheter (16.6%), and two transgastric catheter internalizations (33.4%). In the two cases of total PDO, a double J biliary stent and a double J urological catheter were used.
The approach for the internal drainage prostheses was percutaneous under fluoroscopic-endoscopic guidance. The mean duration of the prosthesis was 183 days (20-376). One patient (16.6%) presented with the prosthesis migration with the subsequent recurrence of a 2 cm collection, which did not require treatment. The prosthesis that migrated was the double-J ureteral catheter. The mean hospitalization time was 69 days, and the follow-up time 951 days. There was no mortality (Figure 3).
Discussion
The complications of acute pancreatitis continue to be a challenge despite advances in minimally invasive techniques. Research shows a progressive increase in the effectiveness of percutaneous and endoscopic approaches, especially after the advent of endosonography4,6-8. Faced with the diagnosis of early infectious complications in acute pancreatitis, such as IPN, the percutaneous approach shows effectiveness rates exceeding 60%9,10, while endoscopic necrosectomy, as the only treatment technique, could reach effectiveness of 81%11.
However, when percutaneous drainage is chosen, there are three possible routes for placing the different catheters: retroperitoneal, peritoneal, and transvisceral (Figure 4).
On the one hand, this approach allows placing one or more catheters, making it possible to wash the pancreatic necrosis through them. These hydraulic debrides can be performed in the patient’s bed without anesthesia. On the other hand, transgastric percutaneous drainage is a safe and effective procedure, which has not yielded significant complications or mortality in our series and avoids major, open, or laparoscopic procedures to treat IPN. It represents benefits for the patient, as shown by the PANTER study, in which early open necrosectomy has a higher rate of morbidity and mortality than the step-up approach12.
Currently, endoscopic pancreatic prostheses are the first option for treating PDO. Nonetheless, the transgastric percutaneous approach could be a therapeutic option since it is possible to direct the pancreatic fluid into the gastric cavity after the internalization of the catheter.
Acute pancreatitis guidelines do not recognize the different percutaneous approaches as more effective. However, according to this experience, it is considered that in the event of a complication of IPN treated percutaneously, at least one transgastric catheter should be placed that subsequently allows its internalization to treat PDO if it occurs.
The placed devices included internalization of the multipurpose catheter, prostheses such as the 7 Fr double J plastic stent, and urological double J catheter (not recommended due to the complexity of its placement and the possibility of migration). The recommended technique is the internalization of the transgastric catheter under fluoroscopic and endoscopic guidance because it is technically simple, and there is less possibility of catheter migration than other prostheses used. Its effectiveness is probably greater in cases of partial ductal disruption than in total PDO, as noted in this series, where there was recurrence in a case of total PDO due to prosthesis migration.
However, more research and a more significant number of patients are required to reach a meaningful conclusion. It is highlighted that the mortality in the studied group was 0%.
Conclusions
Developing a PDO is a therapeutic challenge. Defining whether it is partial or total makes a difference concerning the possibility of effective treatment, recurrence of collections, and the need for possible surgical resection. The present study made it possible to demonstrate that the internalization of the transgastric percutaneous catheter is a therapeutic option for PDO, with low morbidity and no mortality. Nevertheless, more studies and a more significant number of patients are required to analyze the morbidity and mortality and the effectiveness of this type of treatment through a comparative study of techniques.











 text in
text in