Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad de Odontología Universidad de Antioquia
Print version ISSN 0121-246X
Rev Fac Odontol Univ Antioq vol.24 no.1 Medellín July/Dec. 2012
ORIGINAL ARTICLES DERIVED FROM RESEARCH
Molecular analysis of Sonic hedgehog (Shh) in the etiology of nonsyndromic cleft lip and palate in Chilean case-parent trios
Ramiro J. Rincón R.1; José Suazo2; Rafael Blanco C.2
1 Professor, School of Dentistry, Universidad de Antioquia, Medellín,
Colombia
2 Professors, School of Medicine, Universidad de Chile, Santiago, Chile
SUBMITTED: FEBRUARY 28/2012-ACCEPTED: JUNE 12/2012
Rincón RJ, Suazo J, Blanco R. Molecular analysis of Sonic hedgehog (Shh) in the etiology of nonsyndromic cleft lip and palate in Chilean case-parent trios. Rev Fac Odontol Univ Antioq 2012; 24(1): 110-120.
ABSTRACT
INTRODUCTION: nonsyndromic cleft lip and palate (NSCLP) is one of the most common congenital malformations not only
in Chile but also worldwide. It has a multifactorial inheritance pattern with interaction of several genes and the environment. Several
experimental studies have proven the participation of Sonic hedgedhog (Shh) in the migration process of cells from the neural crest,
in the epithelium-mesenchyme transformation, and in the formation of middle craniofacial structures during embryo development; an
association between Shh variants and NSCLP is probable.
METHODS: the goal of this study was to evaluate both exonic and intronic
regions adjacent to Shh, in a sample of 150 case-parent trios in order to find possible associations with NSCLP. The PCR-RFLP method
was used to determine the presence of heteroduplex. Afterwards, the Conformation Sensitive Gel Electrophoresis (CSGE) technique was
used to visualize DNA distortion at the heteroduplexes. As an alternative method, a single-nucleotide polymorphism (SNP) analysis was
performed in order to determine NSCLP-Shh associations, by means of these SNPs: rs1233555 and rs1233556, located at the first Shh
intron.
RESULTS: no heteroduplexes were found in any of the analyzed Shh segments in 150 trios; SNP analysis did not show associations
between Shh and NSCLP either.
CONCLUSIONS: this lack of association may be due to the fact that SNP distribution frequency among
Chilean population is different to that of reference populations, or because the number of SNPs analyzed was not sufficient, or even
because this study did not include other Shh regions.
INTRODUCTION
Nonsyndromic cleft lip and palate is a congenital malformation produced by lack of fusion among the structures that generate the upper lip and secondary palate development.1-3 In mammals, by the fourth week the face is formed by the frontal processes surrounded by first and second branchial arches. Later, the frontal process originates medial and lateral frontal-nasal processes, and the first branchial arch originates maxillary and mandibular processes. Nasomedial processes grow more than the lateral ones, fusing with maxillary processes to form the upper lip and the primary palate.4 In the internal section of the primitive mouth, the palatal shelves of the maxillary process rise and fuse at the middle line with the nasal septum to form the secondary palate.5, 6 Any disruptions during the fusion of these embryo structures produce fissures.7, 8 This pathology is classified as syndromic7 and nonsyndromic cleft lip and palate (NSCLP, OMIM 119530).
NSCLP shows the characteristics of a complex hereditary disease produced by genetic and environmental factors, which create phenotypic expression variability.9 NSCLP is the most frequent worldwide. Amerindian and Asian races are the ones with the most rates of NSCLP.10, 11 In Chile, NSCLP presents an average rate of 1.5 per 1.000 alive newborns, and it is most frequently found among males.12 The Chilean population of lower socioeconomic status presents a greater composition of Amerindian ethnic groups and higher NSCLP rates.13, 14 This anomaly has become a public health problem in Chile due to its social and economic impacts, as it increases its attention and rehabilitation costs.
The following processes are implicated in the development of upper lip and palate: cell migration from the Cranial Neural Crest (CNC),15, 16 epithelial-mesenchymal transformation,17 and formation of middle craniofacial structures.18-20 These processes are regulated by the expression of transcription factors and secretionor cell-surface molecules.2, 9, 17, 21-25 Sonic hedgedhog (Shh) is a form of signaling probably associated to the formation of upper lip and palate.9, 21, 23, 24, 26, 27
Studies on fish19, 27 and mice28 have shown that alterations or deficiencies in Shh block migration of CNC, producing phenotypes similar to NSCLP in humans. It has been observed that Shh in mice is associated to the epithelial-mesenchymal transformation, creating palatal fissures when Shh is mutated.23 In chicken5, 29 and fish,30 Shh alterations produce deficient middle craniofacial structures –phenotypes that are compatible to the holoprosencephaly syndrome,31, 32 along with labial-palatal fissures.
In humans, the Shh gene is located on the 7q-36,3 chromosome region, it is constituted by three exons and two introns, and has a length of 29,4 kb3. Molecularly, the sonic hedgehog (Shh)33 protein behaves like an intercellular signaling molecule, which is synthetized by a precursor that suffers self-catalytic reaction,34 and both cholesterol and palmitic acid covalently adhere to Shh.34-38 It is believed that addition of lipids, cholesterol, and palmitic acid constrains Shh mobility in extracellular environments.39, 40 Sonic hedgehog is essential to the normal development of many organs, and it is a factor for holoprosencephaly.34, 41 Shh is expressed in the epithelium of the branchial arches.42, 43 Shh alterations in mice lead to phenotypes similar to that of NSCLP.8, 23
These antecedents lead to the hypothesis that Shh would be associated to the NSCLP phenotype in humans. To verify this hypothesis, a sample of caseparent trios was used in order to identify alleles, genotypes, and haplotypes transmitted from parents to their descendants and therefore to establish ShhNSCLP association.
MATERIAL AND METHODS
Patients sample. Once all the participants signed a consent form, previously approved by the Ethics Committee of Universidad de Chile's School of Medicine. DNA was extracted from peripheral blood samples of 150 male patients suffering NSCLP and from their parents by using the modified protocol described by Maniatis.44 NSCLP was diagnosed by a geneticist.
Primers were designed to amplify eight segments between 200 and 400 pb (table 1) of the exonic and intronic regions adjacent to Shh exons. These segments were amplified by means of PCR. The Primer 3 software was used to design primers.
The amplified samples were denaturalized at 94 °C and annealed at 64 °C. Possible mutations during the annealing step form mismatch creating deformations in the conformation of amplified segments which are detected by gels sensitive to these deformations, MDE-CSG gels.
In case of finding heteroduplexes, sequencing of the respective amplified segment was performed.
SNP analysis. SNP analysis was an alternative to heteroduplex analysis. Two SNPs were analyzed and their selection was performed by using the NCBI database and the International Project HapMap, having into account their frequency among Europeans and Asians, given the ethnic composition of Chilean population. The software DNA for Windows was used to find the restriction enzymes of these two SNPs. Also, the four primers for amplification of these two SNPs were designed. In order to determine presence of these two SNPs in our case-parent trios samples, we used NlaIII restriction endonucleases for rs1233 555 and Sau3A1 for rs 1233556 (table 2) .
Statistical analysis. Analysis of alleles, genotypes, and haplotypes was performed in order to determine Shh-NSCLP associations, by means of TransmissionDisequilibrium Test (TDT), odd ratio, and likelihood function. The UNPHASED software was used for these analyses.
RESULTS
In order to establish Shh-NSCLP associations, a case-parent trio analysis was performed to identify alleles, haplotypes, and genotypes transmitted from parents to children, by means of the heteroduplex technique. In the sample of 150 case-parent trios, analysis of MDE-CSG gels did not show bands delayed in their migration.
Absence of delayed bands means absence of heteroduplex, suggesting that no mismatches were detected on the amplified segments of the three analyzed exonic and intronic regions adjacent to Shh (figure 1) .
These two SNPs are located in intron 1 of Shh (the first fuchsia box from left to right in figure 2). They were selected due to their high frequency in Asian and European populations by using the National Center for Biotechnology Information (NCBI) database and the International Project HapMap. Shh: Sonic hedgedhog.
The alleles of SNPs rs1233555 and rs1233556 were C > T and C > T, respectively. figures 3A and 3B show the results of NlaIII and Sau3A1 restriction endonuclease enzyme digests on nine amplified segments of SNP rs1233555 and eight amplified segments of SNP rs1233556 respectively.
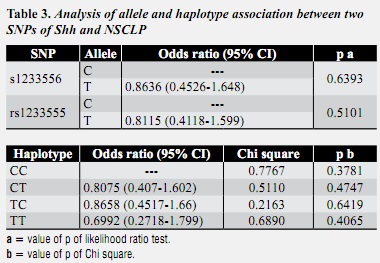
NlaIII digest of the amplified segments of SNP rs1233556 may be observed in the agarose gel of figure 3A; the obtained genotypes were CT, TT and CC. Sau3A1 digest of the amplified segments of SNP rs1233555 may be observed in the agarose gel of figure 3B; the obtained genotypes were CT and CC. Combination of genotypes of both SNPs resulted in haplotypes CC, CT, TC and TT. These haplotypes did not show significant results in terms of association to NSCLP (table 3).
The statistical analysis of alleles, genotypes, and haplotypes did not show their preferential transmission from parents to the affected progeny. These results suggest that no linkage disequilibrium was detected between the two SNPs analyzed in this study. Therefore there was not detected an association between NSCLP and the selected markers (table 3).
DISCUSSION
The purpose of this study was to demonstrate that Shh is associated to NSCLP.
Initially, we used MDE-SGS gels but did not observe bands delayed to ADN migration of eight segments of Shh of 150 trios. This led to the analysis of two SNPs on the first intron of this gene, which presented experimental evidences in the development of upper lip and palate. Analyses of the SNPs did not show Shh-NSCLP associations.
This lack of association between gene Shh and NSCLP among alleles, genotype and haplotype may be due to the fact that the analyzed regions are not involved in such association, but maybe the nonanalyzed regulatory regions up and downstream have a direct or indirect influence on Shh-NSCLP associations. This influence has been suggested by experimental studies which demonstrated that Shh is implicated in cell migrations from Cranial Neural Crest Cells (CNCC), in the formation of middle cranial structures, and in the epithelial-mesenchymal transformation. These events intervene in the differentiation and formation of embryo structures that originate the upper lip and palate.
Mutations and haploinsuficiencies of this gene in fish, chicken, and rats alter cell migration from the cranial neural crest or the positional induction of cranial ecto-mesenchyme cells, affecting the mechanisms of induction, initiation, and execution of specific programs of differentiation among CNCCs and generating phenotypes similar to that of NSCLP in humans.16, 23, 27 But mutations of Shh are not the only ones that influence these conditions, there are also the ones modulated by transcriptional regulators which activate several genes that interact with Shh.
For instance, at a later stage of migration and induction of CNCCs, interaction of Shh with fibroblast growth factor 10 (FGF10) affects epithelial-mesenchymal transformation, a key process in the production of labial-palatal fissures.7 Another example is the case of bone morphogenetic protein 4 (BMP4), that induces Shh expression in the epithelium of the medial edge epithelia of the palatal shelves enabling their growth and fusion.21 Therefore, mutations or functional deficiencies of these Shh-interacting genes may alter such relations and produce the NSCLP phenotype, even if the Shh is not affected. It is also probable that slight Shh modifications severely influence regulation of other genes that participate in the etiology of NSCLP. These regulations of Shh by other genes or vice versa indicate an epistatic relation among these genes in the etiology of NSCLP.45, 46 Another important aspect to consider is the presence of this gene in the holoprosencephaly syndrome in humans, suggesting that Shh would be critical for craniofacial development, depending on the embryo's stage and environment. Shh severe mutations or during early embryo stages could lead towards syndromes accompanied by labial-palatal fissures, while mutations or weak interactions with other genes in advanced embryo stages would result in NSCLP only. We could therefore speculate that mutations or haploinsuficiencies of Shh are not the only requisites for producing NSCLP. Nevertheless, in disagreement with the present study, Orioli et al found out Shh mutations and polymorphisms associated to NSCLP.47 It is possible, however, that sample size and a rigorous control of some factors inherent to the Chilean population were the factors that produced different results in terms of ShhNSCLP associations among the study populations in both studies.
Similarly, maybe the number of SNPs used was insufficient, or it was necessary to include other SNPs located at Shh-regulating regions in order to determine associations of this gene with NSCLP, or distribution of these SNPs in Chilean population present a frequency that differs from that of the reference populations, so it impeded clearly detecting a possible role of Shh in NSCLP.
Nevertheless, it is necessary to analyze cisregulatory regions and Shh-promoting regions which could also contain variants that influence the NSCLP phenotype.
We could then conclude that this lack of association may be due to the fact that SNP distribution frequency among the Chilean population differs from that of reference populations, or that the number of SNP used was not sufficient, or that other nonanalyzed Shh regions needed to be included.
CORRESPONDING AUTHOR
Ramiro J. Rincón R.
School of Dentistry
Universidad de Antioquia
Email address: ramirojrr@gmail.com
REFERENCES
1. Murray JC. Gene/environment causes of cleft lip and/or palate. Clin Genet 2002; 61(4): 248-256. [ Links ]
2. Helms JA, Cordero D, Tapadia MD. New insights into craniofacial morphogenesis. Development 2005; 132: 851-861. [ Links ]
3. Chai Y, Maxson RE. Recent advances in craniofacial morphogenesis. Dev Dyn 2006; 235: 2353-2375. [ Links ]
4. Radlanski RJ, Renz H. Genes, forces, and forms: mechanical aspects of prenatal craniofacial development. Dev Dyn 2006; 235(5): 1219-1229. [ Links ]
5. Hu D, Marcucio RS, Helms JA. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development 2003; 130(9): 1749-1758. [ Links ]
6. Vasiri Sani F, Hallberg K, Harfe BD, McMahonc AP, Lindea A, Gritli-Linde A. Fate-mapping of the epithelial seam during palatal fusion rules out epithelial-mesenchymal transformation. Dev Biol 2005; 285(2): 490-495. [ Links ]
7. Murray JC, Schutte BC. Cleft palate: players, pathways, and pursuits. J Clin Invest 2004; 113(12): 1676-1678. [ Links ]
8. Rice R, Spencer-Dene B, Connor EC, Gritli-Linde A, McMahon AP, Dickson C et al. Disruption of Fgf10/Fgfr2bcoordinated epithelial-mesenchymal interactions causes cleft palate. J Clin Invest 2004; 113(12): 1692-1700. [ Links ]
9. Gritli-Linde A. Molecular control of secondary palate development. Dev Biol 2007; 301(2): 309-326. [ Links ]
10. Tolarová MM, Cervenka J. Classification and birth prevalence of orofacial clefts. Am J Med Genet 1998; 75(2): 126-137. [ Links ]
11. Vanderas AP. Incidence of cleft lip, cleft palate, and cleft lip and palate among races: a review. Cleft Palate J 1987; 24(3): 216-225. [ Links ]
12. Nazer J, Aravena T, Cifuentes L. Malformaciones congénitas en Chile: un problema emergente (periodo 1995-1999). Rev Med Chile 2001; 129(8): 895-904. [ Links ]
13. Palomino HM, Palomino H, Cauvi D, Barton SA, Chakraborty R. Facial clefting and Amerindian admixture in populations of Santiago, Chile. Am J Hum Biol 1997; 9(2): 225-232(a). [ Links ]
14. Palomino H, Cerda-Flores RM, Blanco R, Palomino HM, Barton SA, De Andrade M et al. Complex segregation analysis of facial clefting in Chile. J Craniofac Genet Dev Biol 1997; 17(2): 57-64(b). [ Links ]
15. Santagati F, Rijli FM. Cranial neural crest and the building of the vertebrate head. Nat Rev Neurosci 2003; 4(10): 806-818. [ Links ]
16. Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordial. Genes Dev 2004; 18(8): 937-951. [ Links ]
17. Kang P, Svoboda KKH. Epithelial-Mesenchymal transformation during craniofacial development. J Dent Res 2005; 84(8): 678-690. [ Links ]
18. Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995; 203: 253-310. [ Links ]
19. Brand M, Heisenberg C-P, Warga RM, Pelegri F, Karlstrom RO, Beuchle D et al. Mutations affecting development of the midline and general body shape during zebrafish embryogenesis. Development 1996; 123: 129-142. [ Links ]
20. Schilling TF, Kimmel CB. Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development 1997; 124(15): 2945-2960. [ Links ]
21. Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen Y. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and SHH signaling in the regulation of mammalian palatogenesis. Development 2002; 129(17): 4135-4146. [ Links ]
22. Zucchero TM, Cooper ME, Maher BS, Daack-Hirsch S, Nepomuceno B, Ribeiro L et al. Interferon regulatory factor 6 (IRF6) gene variants and the risk of isolated cleft lip or palate. N Engl J Med 2004; 351(8): 769-780. [ Links ]
23. Rice R, Connor E, Rice DPC. Expression patterns of hedgehog signalling pathway members during mouse palate development. Gene Expr Patterns 2006; 6(2): 206-212. [ Links ]
24. Haworth KE, Wilson JM, Grevellec A, Cobourne MT, Healy C, Helms JA et al. Sonic hedgehog in the pharyngeal endoderm controls arch pattern via regulation of Fgf8 in head ectoderm. Dev Biol 2007; 303: 244-258. [ Links ]
25. Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS. Cdo Functions at multiple points short article in the Sonic hedgehog pathway, and cdo-deficient mice accurately model human holoprosencephaly. Dev Cell 2006; 10(5): 657-665. [ Links ]
26. Helms JA, Kim CH, Hu D, Minkoff R, Thaller C, Eichele G et al. Sonic hedgehog participates in craniofacial morphogenesis and is down-regulated by teratogenic doses of retinoic acid. Dev Biol 1997; 187: 25-35. [ Links ]
27. Wada N, Javidan Y, Nelson S, Carney TJ, Kelsh RN, Schilling TF. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development 2005; 1 32: 3977-3988. [ Links ]
28. Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature 1996; 383: 407-413. [ Links ]
29. MacDonald ME, Abbott UK, Richman JM. Upper beak truncation in chicken embryos with the cleft primary palate mutation is due to an epithelial defect in the frontonasal mass. Dev Dyn 2004; 230(2): 335-349. [ Links ]
30. Takamiya M, Campos-Ortega JA. Hedgehog signalling controls zebrafish neural keel morphogenesis via its leveldependent effects on neurogenesis. Dev Dyn 2006; 235(4): 978-997. [ Links ]
31. Cordero D, Marcucio R, Hu D, Gaffield W, Tapadia M, Helms JA. Temporal perturbations in Sonic hedgehog signaling elicit the spectrum of holoprosencephaly phenotypes. J Clin Invest 2004; 114(4): 485-494. [ Links ]
32. Maity T, Fuse N, Beachy PA. Molecular mechanisms of Sonic hedgehog mutant effects in holoprosencephaly. Proc Natl Acad Sci USA 2005; 102(47): 17026-17031. [ Links ]
33. Varjosalo M, Taipale J. Hedgehog signaling. J Cell Sci 2007; 120: 3-6. [ Links ]
34. Nanni L, Ming JE, Bocian M, Steinhaus K, Bianchi DW, Die-Smulders C et al. The mutational spectrum of the Sonic hedgehog gene in holoprocencephaly: Shh mutations cause a significant proportion of autosomal dominant holoprocencephaly. Hum Mol Genet 1999; 8(13): 2479-2488. [ Links ]
35. Chamoun Z, Mann RK, Nellen D, Von Kessler DP, Bellotto M, Beachy PA et al. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science 2001; 293: 2080-2084. [ Links ]
36. Lee JD, Treisman JE. Sightless has homology to transmembrane acyltransferases and is required to generate active hedgehog protein. Curr Biol 2001; 11(14): 1147-1152. [ Links ]
37. Amanai K, Jiang J. Distinct roles of central missing and dispatched in sending the hedgehog signal. Development 2001; 128: 5119-5127. [ Links ]
38. Micchelli CA, The I, Selva E, Mogila V, Perrimon N. Rasp, a putative transmembrane acyltransferase, is required for Hedgehog signaling. Development 2002; 129(4): 843-851. [ Links ]
39. Gallet A, Rodríguez R, Ruel L, Therond PP. Cholesterol modification of hedgehog is required for trafficking and movement, revealing an asymmetric cellular response to hedgehog. Dev Cell 2003; 4: 191-204. [ Links ]
40. Chen MH, Lin Y-J, Kawakami T, Xu SM, Chuang PT. 2004. Palmitoylation is required for the production of a soluble multimeric Hedgehog protein complex and long-range signaling in vertebrates. Genes Dev 2004; 18: 641-659. [ Links ]
41. Nieuwenhuis E, Hui CC. Hedgehog signaling and congenital Malformations. Clin Genet 2005; 67(3): 193-208. [ Links ]
42. Moore-Scott BA, Manley NR. Differential expression of Sonic hedgehog along the anterior-posterior axis regulates patterning of pharyngeal pouch endoderm and pharyngeal endoderm-derived organs. Dev Biol 2005; 278(2): 323-335. [ Links ]
43. Yamagishi C, Yamagishi H, Maeda J, Tsuchihashi T, Ivey K, Hu T et al. Sonic hedgehog is essential for first pharyngeal arch development. Pediatr Res 2006; 59(3): 349-354. [ Links ]
44. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1989. [ Links ]
45. Ferguson MWJ. Craniofacial malformations: towards a molecular understanding. Nature Genet 1994; 6: 329-330. [ Links ]
46. Ito Y, Yeo JY, Chytil A, Han J, Bringas P Jr, Nakajima A et al. Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 2003; 130: 5269-5280. [ Links ]
47. Orioli IM, Vieira AR, Castilla EE, Ming JE, Muenke M. Mutacional analysis of the Sonic hedgehog in 220 newborns with oral clefts in a south American (ECLAMC) population. Am J Med Genet 2002; 108(1): 12-15. [ Links ]











 text in
text in