INTRODUCTION
Post-cardiotomy cardiogenic shock is among the most feared complications after cardiac surgery and might require advanced therapies such as ventricular assist devices or cardiac transplant. Increasingly used among these therapies is Extracorporeal Membrane Oxygenation (ECMO) as a bridge to recovery or transplant 1. Although this therapy can improve patient prognosis, it is not free of risks, some examples being cannulated limb ischemia, infection, hemolysis, or bleeding 2.
Acute limb compartment syndrome occurs when the pressure inside the aponeurotic compartment rises and affects blood flow and functionality of the tissues involved. Traumatic injury is the most common cause of this syndrome, but it can also be present in non-traumatic conditions, as is the case with the use of intravascular devices like ECMO cannula placement inside the femoral artery 2.
Deciding the optimal anesthetic approach for emergent surgery in critical and unstable patients can be difficult. Although in most cases general anesthesia is necessary, regional anesthesia could be an alternative to reduce surgical complications, it being the technique that less interferes with homeostasis in critically ill patients 3.
We present the case of a patient who required postoperative ECMO after elective cardiac surgery and also urgent surgical treatment after the development of a compartment syndrome. We describe the use of regional anesthesia together with sedation and respiratory support with high flow nasal cannula.
PATIENT INFORMATION
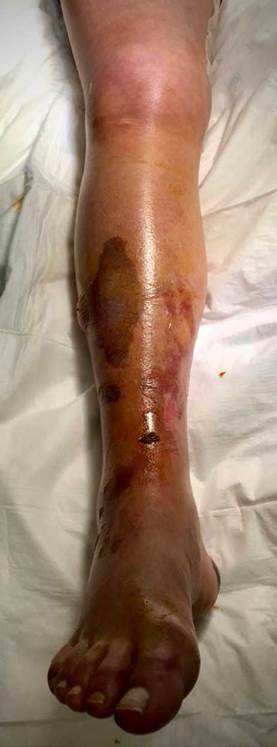
A 57-year-old woman weighing 75 kg, scheduled for mechanical mitral valve replacement, tricuspid annuloplasty and correction of total anomalous pulmonary venous return (TAPVR). Her medical history included moderate-to-severe pulmonary hypertension with mean pulmonary arterial pressure (mPAP) of 48 mmHg and normal pulmonary vascular resistance (PVR) of 2.99 WU evidenced on right heart catheterization, moderate mitral stenosis, severe tricuspid regurgitation, and left recurrent nerve injury of idiopathic origin, which caused left vocal cord paralysis and dysphonia. She was anticoagulated with acenocoumarin and treated with bisoprolol and furosemide (Image 1).
In the operating room, standard monitoring was initiated with invasive arterial pressure, pulmonary artery catheter and regional cerebral oxygen saturation with near infrared spectroscopy (NIRS). General balanced anesthesia was then provided with sevoflurane and remifentanil infusion without complications initially. However, cardiopulmonary bypass weaning at the end of the surgery was unsuccessful due to cardiogenic shock secondary to right ventricular failure despite high doses of norepinephrine, inotropic support with dobutamine and epinephrine, and inhaled nitric oxide. Finally, VA-ECMO was required. The first days in the intensive care unit (ICU) were marked by a slow recovery of cardiac function and decreasing requirements of noradrenaline and dobutamine. ECMO therapy was withdrawn after six days following hemodynamic improvement, and because of suspected right femoral arterial cannula thrombosis, which was confirmed by absent distal pulses and Doppler ultrasound. After removal of the cannula and resolution of the arterial occlusion with open thrombectomy, the patient developed a compartment syndrome requiring urgent surgery to perform a fasciotomy.
The patient was conscious and alert but dyspneic and with increased work of breathing. She required high flow oxygen therapy (HFNT) with FiO2 55%, 36 Lt, alternating with non-invasive bi-level positive pressure ventilation (NIV) to maintain 95% arterial saturation and PaO2/FiO2 around 150mmHg, and she was also on diuretic therapy with furosemide infusion (5 mg/h). Her blood tests showed rhabdomyolysis with CK of 2535 U/L, moderate thrombocytopenia (81,000 per µl), anemia (9.1 g/dL) normal coagulation profile, and normal renal function; anti-Xa level was not measured. The chest X-ray showed diffuse bilateral airspace opacities. She was hemodynamically unstable, requiring dobutamine for inotropic support up to 8 mcg/kg/min, and had paroxysmal episodes of atrial fibrillation. She reported severe pain in the affected limb, which was edematous with red and bright skin.
THERAPEUTIC INTERVENTION
Given the patient's critical condition and past medical history, general anesthesia was discarded because of the high probability of adverse events such as difficult intubation in the context of severely diminished functional reserve, troublesome mechanical ventilation weaning, or hemodynamic instability. Neuraxial anesthesia was not considered because the patient was being anticoagulated with full-dose enoxaparin (80 mg twice a day), with the last dose administered 12 hours before.
Therefore, a bedside ultrasound-guided sciatic nerve block at the level of the popliteal fossa was performed using 1% mepivacaine 30mL and bicarbonate, with no complications. In the operating room, standard monitoring measures were established (continuous ECG, SatO2 and invasive arterial pressure), sedation was initiated with a continuous infusion of dexmedetomidine at 0.6 mcg/kg/h, without initial bolus to preserve the patient's hemodynamics, and up-titrated until a Richmond Agitation-Sedation Scale (RASS) of -1 to -2 was achieved, together with ketamine boluses every five to ten minutes up to a total of 30 mg (0.4 mg/kg). High flow nasal cannula (HFNC) at 36 liters was used and a 55% FiO2 was maintained to keep SatO2 between 91-94%. We decided to continue inotropic support (dobutamine 7-8 mcg/kg/min) titrated to a mean arterial pressure of 65 - 70 mmHg and heart rate between 95 - 105 bpm during the whole intervention.
After achieving the desired level of sedation and correct distribution of nerve blockade, an anterior and lateral fasciotomy of the right tibia was successfully performed with no complications. The patient continued to improve in the ICU with successful weaning from respiratory support. Dobutamine was exchanged three days later for digoxin to improve control of ventricular rate due to atrial fibrillation. Pain was controlled with on-demand morphine. Anticoagulation was resumed the same night of the surgery and the patient was finally discharged from the ICU five days after the fasciotomy.
DISCUSSION
A relatively simple intervention to be performed on a patient with a high risk of perioperative complications is presented in this report. Particular considerations in critically ill patients might contraindicate or at least complicate the anesthetic approach. These include anticoagulation, baseline mental status, or hemodynamic and respiratory support required.
The respiratory situation in our patient was dire since she required high flow oxygen therapy to achieve a PaO2/FiO2 ofi50 mmHg, and she also needed inotropic support with dobutamine. This clinical picture rendered general anesthesia a potentially risky procedure, especially in terms of extubation failure4, severe hemodynamic complications, and prolonged ICU stay.
Under these circumstances, neuraxial anesthesia would be a fine choice; however, low molecular weight heparin (80 mg SQ) had been administered recently as a result of her cardiac surgery and acute arterial ischemia. This was in itself a contraindication for any neuraxial technique unless anticoagulation was reverted or sufficient time had elapsed in accordance with current guidelines (24 hours after full anticoagulation dose), which would have also been unadvisable because of the high thrombotic risk 5.
We opted for an ultrasound-guided sciatic nerve block because of the lowest chance of causing further deterioration of the patient's hemodynamic or respiratory status. Performing a peripheral nerve block also reduces postoperative pain, opioid consumption, and associated respiratory and gastrointestinal side effects. This translates into earlier discharge and a reduction in healthcare costs, and also enables early onset of motor and respiratory rehabilitation, a key factor for complete patient recovery 6. Complications of nerve blockade are rare, most nerve injuries are transient and do not always manifest with clinical symptoms, and ultrasound guidance provides an additional safety margin to avoid intraneural or intravascular injection of local anesthetics.
Sedation with dexmedetomidine was chosen because of its low association with significant impairment of the hemodynamic status or spontaneous ventilation, which needed to be preserved during the procedure 7. Low-dose ketamine boluses up to a total of 30 mg (0.4 mg/kg) were added for additional analgesia and to counteract the hemodynamic effects of dexmedetomidine. In unstable patients, an initial bolus of dexmedetomidine is not advisable due to potential secondary hypotension.
Of utmost importance is to maintain ICU support in the operating room, in this case with dobutamine as inotropic support, and high flow oxygen support.
In conclusion, regional anesthesia along with sedation is an optimal alternative in critically ill patients with a high probability of extubation failure or intraoperative hemodynamic instability. Each case needs to be assessed individually in order to consider distinct anesthesia techniques tailored to the particular conditions of patients admitted to an intensive care unit.
ETHICAL DISCLOSURES
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics commit-tee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).











 text in
text in