INTRODUCTION
Since ancient times, plants have been used by the world's populations in traditional medicine and have become an essential source of treatment, especially in developing countries. The WHO has estimated that 80 % of the world's population depends on plants in primary treatment(Savadi et al., 2020). Nowadays, 25 % of drugs are prepared from medicinal plants, wherein plants are used as a direct or Indirect source in the composition (Ahmed, 2017).
Even today, medicinal plants are still attracting scientists for their minimal side effects and their positive effects on human health(Aye et al., 2019). Indeed, research has focused on the study of natural sources of bioactive compounds. Several extracts, purified fractions, and components were found to exhibit antioxidant, antimicrobial, and antiinflammatory activity (Azab et al., 2016) with little or no toxic effects. These include flavonoids, phenolic acids, glycosides, and tannins (Santos et al., 2016).
These molecules constitute a principal group of secondary metabolites synthetic in plants that provide color and taste to most fruits and vegetables. Even though phenols are considered non-nutritive components, attention is being received due to their several positive effects (Köksal et al., 2017). Therefore, natural compounds are becoming moreprominent in the marketplace since they are considered safe and non-toxic compared to synthetic antioxidants, which have limited due to their various side effects (Shahidi and Ambigaipalan, 2015). For this reason, recent studies focus on the replacement of synthetic antioxidants with natural antioxidants derived from medicinal plants.
Rubia tinctorum (Rubiaceae) is commonly known as madder root, grows naturally in the Mediterranean area, Central Asia, and south-eastern Europe. It has been used since antiquity in industry, in phytotherapy (Lotfollahi et al., 2014), and the treatment of kidney and bladder stones (Markovic et al., 2013). In addition, its high efficacy has not only been seen in medicine but also in the treatment of pathogens such as vine wood diseases, the use of the plant being extremely versatile (Langa-Lomba et al., 2021). It is used as the natural dyeing of textiles in many parts of the world and the food industry as a flavoring agent (Henderson, 2013).
Phytochemical analysis of R. tinctorum has stated richness in the chemical compounds. The most examined are alkaloids, phenol, flavonoids, anthraquinones, cardiac glycosides, tannins, coumarins, vitamins, and minerals (Aboud, 2010).
To the best of our knowledge, although R. tinctorium plants are used to treat several diseases. There are only limited studies on the biological activities of the different parts thereof. Therefore, the main objective of our study was to characterize and compare the phytochemical composition of the aerial and root part extracts. To determine their phenolic contents (polyphenols, flavonoids, tannins). Besides, to evaluate their biological activities (antioxidant and antihemolytic).
MATERIALS AND METHODS
Plant material
Our plant Rubia tinctorum was gathered in June 2018 in the region of Sidi Bel Abbes (Northwest of Algeria). It was identified by Mr. Righi K doctor at department of Biology, Faculty of Nature and Life sciences, Mascara University, Mascara, Algeria. The plant material was dried in the shade in a room for 15 days, then was finely pulverized using an electric grinder.
Preparation of extracts
Ten grams of each powder sample (aerial part and roots) were quenched separately in 100 ml of hydromethanolic solution (water/methanol 20:80 v/v) for 48 hours with stirring to obtain two extracts (root and aerial part hydromethanolic extracts). The extracts were then filtered using a Whatman No. 1 filter paper. All filtrates were pressured to a boiling temperature of 50 °C, then they dried at 40 °C and stored at 4 °C until their analysis (Pharmacognosie, 1999).
Phytochemical screening
Phytochemical analysis of plant extracts is necessary to reveal the presence of certain chemical families. The detection of different phytochemical components of R. tinctorum extracts was performed by chemical detection tests based on phenomena of precipitation or coloration using the methods described in the literature.
Detectionof phenols
To 2 ml of each extract solution, a drop of 2 % FeCl3 alcoholic solution was added. The positive reaction was expressed by the appearance of a green or blue-black coloration(Adou et al., 2016).
Detection of flavonoids
To 0.5 ml of each extract, ten drops of concentrated hydrochloric acid and a few milligrams of sunflower magnesium were added. Positive results were indicated by a pink-red or yellow coloration after three minutes of incubation (Haddouchi et al., 2016).
Detection of tannins
From each extract, 3 ml was taken and placed in a test tube and diluted with chloroform. 1 ml of acetic anhydride was added. Then, 1 ml of sulphuric acid was poured in carefully. A green color was obtained, indicating the presence of tannins (Hossain et al., 2013).
Detection of Saponins
The dry powder was vigorously mixed with water. A positive result was indicated by the appearance of foam for more than 5 minutes (Joshi et al., 2013).
Detection of coumarin glycosids
A few drops of FeCl3 alcoholic solution were added to each extract. The presence of coumarins is indicated by the appearance of a green coloration after the addition of concentrated HNO3 (Joshi et al., 2013).
Detection of anthraquinons
Free anthraquinones: Approximately 0.5 g of the extract was stirred with 5 ml chloroform for ten minutes and filtered. The filtrate was shaken with 5ml of ammonia solution. The presence of pink color in the ammonia phase indicates the presence of free anthraquinones.
Combined anthraquinones: 1 g of powdered extract boiled with 5 ml of10 % hydrochloric acid for five minutes. The cold filtrate was diluted with chloroform. The chloroform layer was then transferred to a test tube. An equal volume of 10 % ammonia was added to the chloroform extract. The presence of combined anthraquinones was indicated by a pink, red, or purple color (Abodunrin et al., 2015).
Detection of alkaloids
0.5 g of the extract was mixed with 5mlof1 % aqueous hydrochloric acid in a water bath. A few drops of Dragendorff's reagent were added to 1 ml ofthe filtrate. Turbidity or precipitation was considered as positive result (Ismail et al., 2016).
Detection of steroids
2 ml acetic anhydride and 2 ml H2SO4 were added to 5 ml of the extract. The color change from violet to blue confirmed the presence of steroids(Kumar Bargah, 2015).
Detection of proteins
2 ml of NaOH (4 %) and a few drops of 3 % CuSO4 solution were mixed with 2 ml of solution extract. The presence of protein is indicated by the formation of purple or pink color (Maria et al., 2018).
Detection of quinones
3 mL of each extract was treated with 3 mL of chloroform and then the chloroform layer was separated. A volume of potassium hydroxide (5 %) was then added to the chloroform layer. The appearance of red color indicates the presence of quinones(Amezouar et al., 2013).
LC-ESI-MS/MS analysis
The aerial part and root hydromethanolic extracts of R.tinctorum wereassessedby LC-ESI-MS/MS analysis. A high-performance liquid chromatography (HPLC) model 1260 Infinity II LC System coupled with a tandem mass spectrometer was used. The separation was performed in the reverse phase on an Agilent Poroshell 120 EC-C18 analytical column (100mmx3.0mm, 2.7 Mm). The elution gradient consisted of eluent: A (water+5 mM ammonium formate) and eluent B (acetonitrile+0.1 % formic acid), the solvent flow rate was set at 0.250 ml/min and the gradient was as follows: 1 min 40 % A-60 %B; 2 min 70 %A - 30 % B; 3 min 70 % A- 30 % B; 4 min 40 % A-60 % B; 5 min 10 % A-90 %B. The injection volume was 5 µL. The temperature of the column was set at 25 °C.
Agilent 6460 Triple Quad Mass Spectrometer System model tandem mass spectrometer and electrospray ionization (ESI) source operating in both negative and positive ionization modes were used.
The collision energies (CE) were optimized to generate optimal phytochemical fragmentation and maximal transmission of the desired productions. The MS operating conditions were applied as capillary temperature 350 ◦ C, nebulizer gas N2 flow 15 L/min, mode source voltage-4400 V.
The MRM was optimized to selectively detect and quantify phytochemical compounds based on the screening ofspecified precursor phytochemical-to-fragment ion transitions.
Quantitative phytochemical characterisation Total phenolic content
The total phenol content of both extracts was assessed by the Folin-Ciocalteu method. An aliquot of 250 µL of the sample (1 mg/mL) and gallic acid (standard) was blended with 1.25 mL of Folin-Ciocalteu reagent recently prepared (1/10) and 1 mL Na2CO3 at 75 g/L. After agitation, the various solutions were incubated at a temperature of 40 °C for 30 min. The absorbance was determined by a spectrophotometer (Shimadzu) at 765 nm. A calibration curve was prepared using a mixture of gallic acid and distilled water at different concentrations of 0.0312, 0.0625, 0.125, 0.25, 0.5, and 1 mg/mL. The results were taken in triplicate and expressed in equivalent milligrams of gallic acid per gram of dry vegetable matter (mg GAE/g) according to the formula (Amezouar et al., 2013):
C = C1* V/m
Where C: total phenolic content in mg GAE/g
C1: Concentration of gallic acid estimated from the calibration curve in mg/mL
V: volume of diluted extract in mL
m: the weight of the plant extract in grams.
Total flavonoids content
The total flavonoid content of the extract was quantifiable as a result of the formation of a complex flavonoid-aluminum using the AlCl3 assay, as described by (Ohikhena et al., 2018) withsome modifications. The protocol is based on the measurement of the quantity of the yellow orange color that is generated when the flavonoid reacts with AlCl3. In brief, a 500 µL aliquot of the extract (1 mg/mL) was added to 150 µL of 5 % sodium nitrite and 2 mL of distilled water. The mixture was vortexed and allowed to stand for 5 minutes. Afterward, 150 µI of AlCl3 (10 %) was added to the solution and left to stand for a further 5 minutes, then 1 ml of 1 M sodium hydroxide was versed to the mixture. The melange was topped to 5 ml with distilled water. The absorbance was then measured at 420 nm using a spectrophotometer. Blanc contains all components except the extract. Catechin standard solution was prepared with varying concentrations (0.0312-1 mg/mL) using the same procedure and expressed in equivalent milligrams of catechin per gram of dry vegetable matter (mg CAE/g Extract). The experiment was carried out in triplicate (Amezouar et al., 2013).
Total tannin content
The content of tannins was evaluated following the procedure previously described by (Hamdi et al., 2018). The experiment was carried out in triplicate. A volume of 50 µL extract (1 mg/mL) was mixed with 3 mL of methanolic vanillin solution (4 % w/v), then 1.5 mL of concentrated H2SO4 was added. The mixture was vortexed and incubated for 15 minutes. Then, the absorbance was measured at 500 nm. The blank was the methanol.TTC reported as mg catechin equivalent per gram dry weight (mg CAE/g).
Evaluation of the antioxidant activity DPPH free radical scavenging assay
The antioxidant activity of extracts was evaluated by the DPPH (1, 1-diphenyl-2-picrylhydrazyl) with the reported method (Rashid et al., 2016). 200 µL of each extract at different concentrations (0.031-1 mg/mL) were added to 2 mL of 0.004 % DPPH solution. The mixture was incubated in the dark for 30 min. Then, the absorbance was measured at 517 nm. The positive control was ascorbic acid. The negative control contained 200 mL of methanol and 2 ml of DPPH methanolic solution. The blank comprised 200 mL of each concentration of the extract or standard and 2 ml of methanol.
The scavenging effect capability was calculated using the following equation:
Scavenging ability in % = (Abs Control - Abs Sample / Abs Control) x 100
Where: Abs of control (DPPH + methanol); Abs sample: Absorbance of sample extract or standard; IC50 values were determined by linear regression analysis.
Ferric reducing antioxidant power (FRAP)
The Frap activity was assessed according to the literature (Amezouar et al., 2013). A mixture of 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide 1 % was mixed with 0.2 mL of diluted samples (0.093-3 mg/ mL) of each extract. The whole was heated at 50 °C for 20 minutes. Then 2.5 mL of trichloroacetic acid (10 %) was added, and the solution was centrifuged for 10 min at 3000 rpm. 2.5 mL of supernatant from each sample was removed and mixed with 1 mL of ferric chloride (1 %) and 2.5 mL of distilled water. Ascorbic acid was used as a positive control. The absorbance was recorded at 700 nm, the high reduction power is presented by the high absorption.
Total antioxidant capacity (TAC)
The anti-radical activity of the aerial part and root extract was evaluated by the phosphomolybdenum assay using the method described by (Idris et al., 2017). 0.3 mL of each sample at various concentrations (0.031-20 mg/mL) was added to 3 mL of reagent (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The whole was heated in a water bath at 95 °C for 90 minutes. The absorbance was measured at 695 nm against a blank (methanol). Ascorbic acid was used as a positive control. The percentage inhibition of the different extracts was calculated according to the formula:
% du TAC = ((Abs 0 - Abs 1) / Abs 0) x 100
Where: Abs 0: (absorbance of the control), A1 (absorbance in the presence of the sample).
In vitro anti hemolytic activity
The antihemolytic activity of the extracts was estimated by the method of Nabavi et al. (2013). Briefly, rat blood was centrifuged at 2000 rpm for 10 minutes, and then it washed with phosphate buffer (pH 7.4) till the supernatant became colorless. The erythrocytes were diluted with phosphate-buffered saline to obtain 4 % suspension. Afterward, an aliquot of 0.5 mL of each extract (0.031-1 mg/ mL) was mixed with 2 mL of erythrocyte suspension (4 %) then the volume was made up to 5 mL with saline buffer. The mixture was incubated at room temperature for 5 min. After incubation, 500 µL of H2O2 was added. It was then slightly stirred and incubated at 37 °C for 240 min. Then the mixture was centrifuged at 1500 r/min for 10 min. The absorbance was recorded at 540 nm. The percentage inhibitory effect was measured according to the same formula used in the DPPH test. In a similar fashion, ascorbic acid was treated.
Data analysis
All determinations were performed in triplicates, and the results were expressed as mean ± SD. The data were analysed using IBM SPSS STATISTICS 23. The relations between the experimental parameters were investigated using one-way ANOVA. Differences were considered statistically significant when p< 0.05.
RESULTS
In the present study, two extracts were assessed for phytochemical composition and biological activities thereof, namely the hydromethanolic root extract and hydromethanolic aerial part extract. These extracts are hereafter termed MR and MF, respectively.
The highest yield extraction percentage of the twoR. tinctorum hydromethanolic extracts was recorded for MR extracts at 17.3 %, whereas MF extracts recorded 16.1 %. The MR seems to be the best part for giving the maximum yield.
Phytochemical screening
Table 1 reports the qualitative results of the phytochemical compounds present in MR and MF extracts. Our data showed the presence of tannins, phenols, flavonoids, coumarin glycosides, and alkaloids in both extracts. However, steroids are absent.
Phenolic compounds profiles
The chemical compounds of the two extracts of R. tinctorum evaluated by LC-ESI-MS/MS are displayed in a table 2. A total of nineteen components were detected and listed in order of elution and retention time. The MR extract represented 15 components. The main compound was citric acid, representing 15.35 µg/mg. Regarding the aerial part, 17 compounds were identified, hesperidin was the major (3.98 µg/mg).
Quantitative phytochemical determination Total phenolic, flavonoids and tannis contents
Catechin and ascorbic acid were used to estimate the total content of polyphenols, flavonoids, and tannins in the two extracts of R. tinctorum. As shown in table (3), the total content of polyphenols, flavonoids, and tannins in the MR extract was 118.38 ± 1.20 mg GAE/g, 45.29 ± 0.04 mg CAE/g, and 42.570 ± 0.088 mg CAE/g, respectively. While the MF extract showed a content of 71.474 ± 0.021 mg GAE/g, 30.305 ± 0.009 mg CAE/g, and 134.1 ± 1.0 mg CAE/g for polyphenols, flavonoids, and tannins, respectively.
Antioxidant activity
To evaluate the antioxidant activity of R. tinctorum extracts three different in vitro assays (DPPH free radical scavenging activity, FRAP assay, and total antioxidant capacity (TAC) methods) were performed. The results are shown in figures (1).
MF extract had the best antioxidant activity in DPPH (83.23 ± 0.007 %) and FRAP (1.51 ± 0.22) tests. Nevertheless, it showed less activity than ascorbic acid and MR in total capacity. On the other hand, it showed comparable results to ascorbic acid (IC50= 0.073±0.02 mg/mL), which requires eight times less energy for inhibiting 50 % of the DPPH free radicals (Table 4). Besides, the antioxidant properties of the MR extract were tested. The result revealed that the extract exhibited no significant radical scavenging activity and reducing ability at the highest concentration tested (1 mg/ mL) compared to the MF. However, a maximum reduction of Mo (VI) to the Mo (V) at 20 mg/mL was noted.
Table 2 Polyphenolic content of the Rubia tinctorum MF and MR extracts analyzed by the LC-ESI-MS/MS technique. MF: Aerial part hydromethanolic extract, MR: Root hydromethanolic extract, ND: not detected, NV: negative, PV: positive
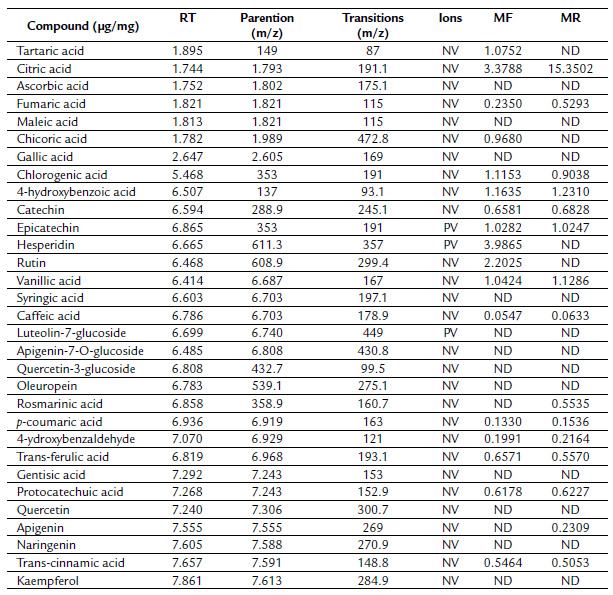
Likewise, the aerial part extract, revealing the existence of 17 phenolic compounds, hesperidin was the major constituent (3.98 µg/mg). Moreover, it is containing other phenolic compounds that are absent in the root extract (tartaric acid, chicoric acid, hesperidin, and rutin).
Nevertheless, it devoid of rosmarinic acid and apigenin. To the best of our knowledge, this is the first study revealing the chemical constituents of R. tinctorum aerial part. Overall, MF extracts gave higher contents of phenolic compounds. However the MR gave the highest concentration.
In vitro Antihemolytic activity
The inhibitory effect of R. tinctorum extract and ascorbic acid against H2O2 is presented in figure 2. Ascorbic acid, MR, and MF exhibited a concentration-dependent manner. Ascorbic acid showed the best antihemolytic effect, while MF had the lowest activity. The calculated IC50 were 0.68±0.002, 1.006±0.10, 0.55±0.004 mg/mL for MR, MF, and ascorbic acid, respectively (Table 3).
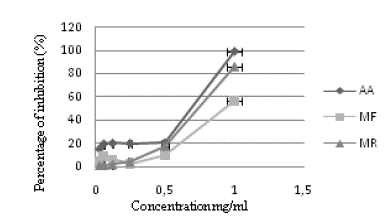
Figure 2 Antihemolytic activity of R. tinctorum extracts. Each value represents a mean±SD (n = 3). MF: Aerial part hydromethanolic extract, MR: Root hydromethanolic extract, AA: Ascorbic acid.
Table 3 Yields, total phenols, flavonoids, tannins content,IC 50 values of anti hemolytic, DPPH, and TAC antioxidant activity of Rubia tinctorum hydromethanolic extracts. MF: aerial part hydromethanolic extract, MR: root hydromethanolic extract, AH: antihemolytique activity, AA: ascorbic acid, TPC: total phenol content, TFC: total flavonoids content, TTC: total tannins content. The data were analysed by one-way anova, values represent mean±SD; n=3. * p<0.05** p< 0.01.

DISCUSSION
Medicinal plants are important in pharmacological research, treatment, and prevention of diseases, as a medical compound, and as raw materials for the preparation of pharmacologically active products(Carvalho et al., 2016). Many drugs nowadays are basedon natural sources obtained from a medicinal plant. Rubia tinctorum is among the plants having several biological activities (Eltamany et al., 2020).
In this study, the phytochemical composition of the hydromethanol extracts of R. tinctorum was assessed. Moreover, their antioxidant, antibacterial, and anti-hemolytic activities were investigated.
Table 1 reports the qualitative results of the phytochemical compounds present in MR and MF extracts. Our data showed the presence of tannins, phenols, flavonoids, coumarin glycosides, and alkaloids in both extracts. However, steroids are absent. The findings of our study are consistent with those of (Aboud, 2010), that demonstrated the presence of the same constituents except for saponin in the hydromethanolicextract of R. tinctorum.
The difference in the components of the two parts is, according to some authors, related to several factors. Among them, the morpho-anatomical structure, the physiological activity of the different parts of the plant, geographical factors, climatic conditions, and gene expression (Iloki-Assanga et al., 2015; Szakiel et al., 2011).
To examine the phenolic composition of hydromethanolic extracts, LC-ESI-MS/MS analysis was carried out. Table 2 shows the polyphenolic content of the R. tinctorum MF and MR extracts. The result revealed that 19 phenolic compounds were detected, including 15 were presented in the root extract. In addition, Citric acid was the main compound (15.35 Jg/mg) therein. Interestingly, this compound has not been identified previously in R. tinctorum.
The identification of the main constituents of R. tinctorum by LC-MS/MS and LC-HRMS was previously performed(Lajkó et al., 2015; Eltamany et al., 2020; Langa-Lomba et al., 2021), revealing the presence of many anthraquinones as munjistine, rubyric acid, lucidine, rubiadine, pseudopurpurine, and nordamnacanthal. Moreover, other studies revealed that quercetin, rutin, hesperidin, and kaempferol as the main phenolic compounds in alcoholic extracts of Rubia tinctorum (Derksen et al., 2002; Eltamany et al., 2020). These differences noted might be due to the genetics, ecology, harvest conditions, different extraction methodology, and environmental conditions (Rached et al., 2019).
It is well known that these molecules have many biological activities. Indeed, it has been previously reported as having excellent effectiveness as an anti-inflammatory and antioxidant (Kadam et al., 2018). Chlorogenic acid is one of the phenolic compounds found in our analysis. It has been shown to have an anxiolytic and antioxidant effect (Bouayed et al., 2007). Another study reported by (Cemeli et al., 2009)revealed the protective effect of gallic acid and rutin against the harmful effects of H2O2. (In et al., 2013) explained the antimicrobial effect of citric acid against Shigella. Epicatechin is another compound in the extract. It possesses many biological activities. Notably antioxidant, antitumor, anti-inflammatory, anti-diabetic activity, antimicrobial, and cardioprotective activity.
Polyphenolic compounds are documented as good natural antioxidants that act as scavengers of radical or metal chelating agents (Louli et al., 2004). For this reason, the quality polyphenolic of both extracts was assessed. The total phenolic, flavonoid, and tannins contents of R. tinctorumwere estimated using the Folin-Ciocalteu reagent and aluminum chloride methods.
Table 3 illustrated the results of total phenols, flavonoids, and tannins content of root and aerial part hydromethanolic R. tinctorum extracts. It is evident from the results obtained that the roots of R. tinctorumwere higher in total phenolic compounds (118.38±1.2 mg GAE/g) than the aerial parts (71.474±0.021 mg GAE/g), while the aerial extract was characterized by its higher content of condensed tannins (134.1±1.0 CAE/g). In addition, the root part extract contained significantly higher amounts of flavonoids (45.29±0.04 mg CAE/g) than the MF extract (30.305±0.009 mg CAE/g). Based on these results, we can suggest that the roots are a good source of phenols and flavonoids, whereas the aerial parts are a source of tannins.
Our findings are in line with previous research. (Rovcanin et al., 2015)found that tannins values in R. tinctorum root were 6.2 mg GAE /g dry extract, and the level of phenols and flavonoids was 14.7 mg GAE/ g, 1.1 mg of CE/g of extract, respectively. Essaidi et al. (2017) reported a higher content of total phenolic and flavonoids compounds in the hydrolysed extract of R. tinctorum root. The same work confirmed the highest content of phenols than the flavonoids in non hydrolysed extract prepared with 80 % methanol (38.84±0.6 mg EGA/g; 13.41±0.34 mg QE/g, respectively) (Essaidi et al., 2017). Similarly, (Marhoume et al., 2019) found that the total polyphenol content of butanolic root extract was 1.1±0.041 mg GAE/100 g DM while the total flavonoid content was 0.7±0.0055 mg CAE/100 g DM. It seems that these differences observed in the amount of phenolic compounds can be due to the plant maturity, stages of the plant growth, biochemical and structural processes of the plant tissue, and the harvest season (Cezarotto et al., 2017).
To determine the antioxidant activity of R. tinctorum, three methods were used (the free radical scavenging activity, the ferric reducing power activity, and total antioxidant capacity).
The free radical scavenging activity of R. tinctorum extracts was tested using the DPPH method. As shown in Figure 1a., MF extract showed the highest antioxidant activity (83.23±0.007 %; p< 0.001) compared to MR extract (66.17±0.02 %). Furthermore, as demonstrated in table 3, a standard reference (ascorbic acid) exhibited an eightfold greater degree of free radical inhibition for DPPH (IC = 0.073 mg/mL) as compared to MR extract (IC = 0.68 mg/mL) and MF (IC 50 = 0.60 mg/mL). Few studies were found in the literature on antioxidant activity of the R. tinctorum plant. However no report on DPPH activity was found for aerial and root parts thereof. Thus, they were not easily comparable.
The difference in DPPH radical scavenging activities of the two extracts was possibly due to a chemical interaction in that the phenolic compounds donate the H atom to the DPPH radical. This interaction depends on the structural conformation of phenolic compounds (the number of available hydroxyl groups) and the structural features such as energy-related O-H dissociation. In addition, the resonance delocalization of the antioxidant and stereochemistry(Vadivel and Biesalski, 2011). Due to the DPPH radical scavenging activity of the extracts, a stable molecule is formed (Yen y Wu, 1999).
The data of the reducing power activity of extracts are illustrated in Figure 1b. The findings were similar to those obtained for the DPPH assay. The MF extract had higher absorbance values (1.51±0.22) than that of the MR (0.31±0.05). Besides, the ascorbic acid had a much higher FRAP value (2.74±0.33: p<0.05) compared to both extracts. Our findings are consistent with those of other studies (Essaidi et al., 2017) and (Siva et al., 2011)on the root extract of R. tinctorum, demonstrating the ability of this plant to reduce ferric ions.
The presence of phenolic and flavonoids compounds in higher concentrations in the root and aerial part extracts provide a possible explanation for the obtained results. Indeed, Afsar et al. (2016) reported that the high amount of polyphenols in extracts could be responsible for their ferric reducing power.
Regarding the total antioxidant activity, it was assessed by using phosphomolybdate. In the presence of antioxidants, Mo (VI) is reduced to the Mo (V) form, thereby forming a complex of green phosphorus and molybdenum V at acid pH (Prieto et al., 1999). The data are displayed in Figure 1c. The MR extract showed the highest reduction of Mo (VI) activity in a dose-dependent manner (61.09%; p<0.01) with a marginal effect than ascorbic acid (95.35 %). While the MF showed a lower reducing power (10.23 %). Our results imply that R. tinctorum could reduce Mo (VI) to Mo (V) through phytoconstituents that are present therein. Such as flavonoids and related polyphones (Sharififar et al., 2009; Khan et al., 2012;). Furthermore, the quantitative analysis revealed that a higher content of polyphenolic compounds was found in the MF extract. However, the highest antioxidant capacity was detected for MR, indicating that the antioxidant activity of the TAC could be attributed to another non-phenolic compound. It is pointed out by some authors that the phosphomolybdenum assay is generally related to other antioxidant substances. Such as vitamins, carotenoids, a-tocopherol, cysteine, and aromatic amines for their ability to donate hydrogen and electrons (Afsar et al., 2016). Taken together, the antioxidant activity effect of R. tinctorum varies depending on the part of the plant and the nature of phytochemical compounds present in each part plant.
The most abundant cells in the human body are the erythrocytes.They are considered as the first target free radicals that can cause hemolysis of red blood cells. In this experiment, we assessed whether MR and MF extracts prevented the hemolysis of erythrocytes. To the extent of our knowledge, this is the first study demonstrating the antihemolytic effect of Rubia tinctorum.
According to our results (Fig. 2), the inhibition percentage of MR extract was slightly lower (1.17- 85.99 %) than that of ascorbic acid (14.85- 98.75 %, p < 0.05). Besides, MF exhibited the weakest antihemolytic activity (57.05 %) at 1mg/ml compared to ascorbic acid and MR.
The findings of the present study demonstrate the presence of antioxidants that have an antihemolytic effect. The bioactive compounds present in both extracts could be responsible for inhibiting the hemolysis of red cells by H2O2. Indeed, several previous research has shown that the presence of polyphenols and flavonoids in crude extracts enhances the stability of the red blood cells membrane, thereby inhibiting hemolysis (Chaudhuri et al., 2007).
CONCLUSIONS
This research highlights the phytochemical composition, antioxidant and anti-hemolytic activities of extracts from the aerial and root parts of R. tinctorum. The results showed that both parts of R. tinctorum can be a source of bioactive compounds. The MR and MF extracts contain 15 and 17 different components, respectively. Citric acid represents the main component. The MF extract has the best antioxidant activity, while the MR seems to have the best anti-hemolytic effect. According to the literature, the study of the aerial part was carried out for the first time. Thus, it will enrich the literature.
Overall, the findings suppose that the aerial and root parts of R. tinctorum could be a source of natural antioxidants for use in the food and pharmaceutical industries. Nevertheless, further studies are needed to explore the potential and isolate the active compounds responsible for these biological activities. As well as in vivo studies are required to assess the toxic anti-hemolytic effect of R. tinctorum.

















