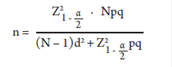Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.32 no.3 Bogotá July/Sept. 2017
https://doi.org/10.22516/25007440.152
Original articles
Precursor Lesions of Malignant Gastric Cancer and Associations with Eating Habits
1Mg. en Epidemiología, Universidad CES; directora del Programa de Nutrición y Dietética, Universidad Mariana, San Juan de Pasto (Colombia). Correo electrónico: yyepez@umariana.edu.co
2Mg. en Epidemiología, Universidad CES; jefe de Programas y Proyectos, Alcaldía Municipal Sibundoy, Sibundoy, Putumayo (Colombia). Correo electrónico: paricaurte@unicauca.edu.co
3Director del Centro de Investigaciones de Enfermedades Digestivas y Nutricionales del Hospital Universitario Departamental de Nariño, San Juan de Pasto, Nariño (Colombia). Correo electrónico: alvarobedoya2@yahoo.es
4PhD en Epidemiología y Bioestadística; docente de la Universidad CES de Medellín, Medellín (Colombia). Correo electrónico: dberbesi@ces.edu.co
Introduction:
Associations between dietary habits and precursor lesions of gastric malignancies including chronic atrophic gastritis, intestinal metaplasia and mild dysplasia were identified in men and women between the ages of 30 and 60 who came to the gastroenterology department of the Center for Research on Digestive Diseases of the city of Pasto, Nariño in the last quarter of 2015 and the first half of 2016.
Methodology:
This is a cross-sectional analytical study in which histological, social, demographic, anthropometric and dietary variables were analyzed. Descriptive, bivariate and multivariate analyzes were performed using crude and adjusted odds ratios with a 95% CI.
Results:
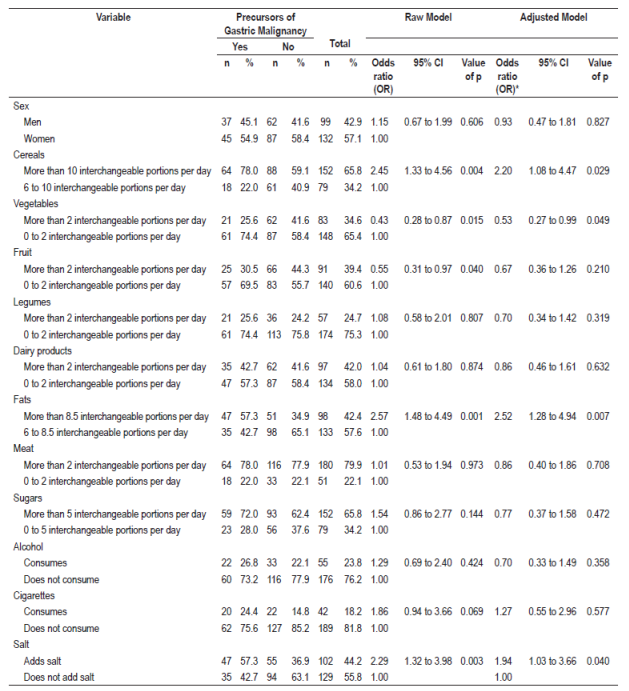
Of the 231 patients surveyed, 35.5% were diagnosed histologically with precursor lesions. The average age was 46 years old, and 57.1% of the cases were women, 32.5% were professionals, and 58% were affiliated with the subsidized health care scheme. These lesions were associated with cereal consumption of above 10 exchanges (OR 2.20, 95% CI: 1.08 - 4.47) and with and fat consumption above 8.5 exchanges (OR 2.52, 95% CI: 1.28-4.94). Adding salt to meals increased the likelihood of developing stomach lesions by 1.94 (95% CI: 1.03-3.66). Regular consumption of vegetables for more than two exchanges reduced the likelihood of stomach lesions (OR 0.53; 95% CI: 0.27-0.99).
Conclusion:
This study aims at contributing knowledge about factors that involved in initiation, promotion and progression of gastric cancer.
Keywords: Precursor lesions of malignant cancer; eating habits; gastric cancer
Introducción:
se determinó la asociación entre los hábitos alimentarios y la presencia de lesiones precursoras de malignidad gástrica (gastritis crónica atrófica, metaplasia intestinal y displasia leve), en hombres y mujeres entre los 30 y los 60 años de edad que acudieron a consulta de gastroenterología en el Centro de Investigación de Enfermedades Digestivas de la ciudad de Pasto (Nariño) durante el último trimestre del año 2015 y el primer semestre del año 2016.
Metodología:
estudio analítico transversal en el que se analizaron variables histológicas, sociales, demográficas, antropométricas y alimentarias. Se realizó un análisis descriptivo, bivariado y multivariado por medio de la odds ratio (oportunidad relativa) cruda y ajustada con un IC del 95%.
Resultados:
el 35,5% de los 231 pacientes encuestados fueron diagnosticados histológicamente con dichas lesiones; la edad promedio no superó los 46 años; predominó el sexo femenino con 57,1%; el 32,5% de los pacientes tenía una formación de nivel profesional y el 58% estaba afiliado al régimen subsidiado. Estas lesiones se asociaron con el consumo de cereales y grasas por encima de los 10 y 8,5 intercambios (OR 2,20; IC del 95%: 1,08-4,47 y OR 2,52; IC del 95%: 1,28-4,94), respectivamente. La incorporación de sal a las comidas servidas aumenta la probabilidad de presentar lesiones estomacales en 1,94 (IC del 95%: 1,03-3,66). El consumo regular de vegetales por encima de los 2 intercambios reduce la probabilidad de presentar lesiones estomacales (OR 0,53; IC del 95%: 0,27-0,99).
Conclusión:
con la investigación, se pretende aportar conocimientos acerca de diferentes factores que pueden participar en la iniciación, promoción y progresión del cáncer gástrico.
Palabras claves: Lesiones precursoras de malignidad; hábitos alimentarios; cáncer gástrico
Introduction
Gastric cancer is a public health priority. Its incidence ranks fifth among cancers after lung, breast, colorectal and prostate cancer, but its mortality rate ranks third after lung and liver cancer. More than 73% of cases of gastric cancer occur in Asia, Europe contributes almost 15% of the global burden, while Central and South America contribute only 7%1. In the department of Nariño, especially in the Andean region, where 81.7% of the department’s population is located, an incidence of gastric cancer of 150 cases per 100,000 inhabitants has been reported. The world’s medical literature indicates that any population with an incidence exceeding 20 per 100,000 inhabitants is considered to be a high risk area 2.
Infections by Helicobacter pylori, changes in the epigenetic mechanisms of gene regulation, diet, and consumption of alcohol and tobacco are considered to be the main factors in the development of gastric cancer. 3,4 A sequential model has been established for the study of this pathology. It explains the progression of gastric carcinogenesis during which normal mucosa undergoes alterations such as superficial gastritis, atrophy, loss of glands, intestinal metaplasia and, finally, dysplasia. At first, intestinal metaplasia resembles the histology of the small intestine, then the large intestine. Together these are known as precursor lesions for gastric malignancy. 4,5 Currently, about a third of all cases of gastric cancer have been attributed to diet which is why study of the etiopathogenesis of chronic diseases has become increasingly important. Hence the importance of assessing food consumption and analyzing nutrient consumption to predict the health status of a community and guide preventive actions. 6
The objective of this study was to determine associations between dietary habits and development of precursor lesions of gastric malignancy in people who were seen in the gastroenterology department of the Digestive Disease Research Center of the city of Pasto.
Methodology
This is an analytical cross-sectional study. It analyzes social, demographic, anthropometric and alimentary variables as well factors such as smoking, consumption of alcohol, use of salt in foods eaten and histological variables in order to diagnose the presence or absence of precursor lesions of gastric malignancy.
We surveyed 231 patients referred by several health care institutions for endoscopy at the Digestive Disease Research Center of the city of Pasto. The non-probabilistic sampling method was used to select the sample based on the accessibility criterion. We took the frequency of lesions of 38.6% reported for Pasto in 2010 into account 2 to determine sample size on the basis of a population of 630 people which is the average number of patients examined in this center over the last 7 years and in order to achieve accuracy of 5%. The sample was calculated by the following formula:
n = Sample size
N = Size of the population defined as the average number of subjects treated at the Digestive Disease Research Center in the last 7 years
Z = Value of the Gaussian distribution 1.96 for α = 0.05
p = Frequency of precursor lesions of gastric malignancy
q = 1 - p
d = Maximum permissible error (5%)
A consumption frequency questionnaire was used to collect information. It featured a closed list of foods that are typical of this region and which were grouped according to nutritional value rather than by place of origin. With this method, foods belonging to each group are interchangeable and the values of energy and macronutrients are kept constant according to statistical principles of variability and homogeneity. This is true as long as standardized portions are used for each food item. This is known as the interchangeable food portions system. Through this system, a more accurate estimate was obtained for the daily number of interchangeable portions consumed for each food group in a day. The data was collected by the project researchers and research assistants who had received training and standardization regarding the application of the instrument. Information was collected before digestive endoscopy was performed.
Stata version 10.1 for Windows was used for the statistical analysis. A descriptive analysis was done, and measures of central tendency were calculated for quantitative variables. Dispersion or position were calculated depending on the distribution of the data. Qualitative variables were summarized with frequency measurements. The crude and adjusted odds ratios were calculated with their respective 95% confidence intervals (95% CI) through binary logistic regression. Variables that have been reported as possible risk or protective factors were included in the adjusted model. Values of p less than 0.05 were considered to be significant.
This investigation is considered to be without risk in accordance with Resolution 008430 of 1993 of the Ministry of Health of Colombia. The variables included in the survey did not modify or deal with sensitive aspects of behavior. The study was approved by the Human Research Ethics Committee of CES University (Act No. 81 of September 28, 2015, project code: 481).
Results
Of the 231 patients surveyed, 35.5% were diagnosed histologically with precursor lesions of gastric malignancy, the average age did not exceed 46 years, the female sex predominated with 57.1%, 32.5% had professional training, and 58% were affiliated to the subsidized health-care regime. These lesions were associated with the consumption of more than 10 interchangeable portions of cereals per day (OR 2.20, 95% CI: 1.08 to 4.47) and more than 8.5 interchangeable portions of fats per day (OR 2.52, 95% CI: 1.28-4.94). Food guides for the Colombian population establish these numbers of interchangeable portions as the maximum recommended amounts of interchangeable portions for these food groups, so they were taken as reference pints for this study. The incorporation of salt into meals increases the probability of lesions of the gastric mucosa by 1.94 times (95% CI: 1.03-3.66). The regular consumption of more than two interchangeable portions of vegetables per day reduces the probability of precursor lesions of malignancy (OR 0.53, 95% CI: 0.27-0.99). Although the crude consumption model indicated that consumption of more than two interchangeable portions of fruit per day was associated with decreased numbers of these lesions (OR 0.55, 95% CI: 0.31-0.97), when the model was adjusted, the association was no longer found (OR 0.67, 95% CI: 0.36-1.26). None of the other factors studied including other food groups, alcohol, cigarettes, and gender showed significant associations with precursor lesions of gastric malignancy (Table 1).
Discussion
Lifestyle factors including diet are recognized as potentially important determinants of increased risk for developing chronic diseases such as cancer. It has been established that 35% of deaths from this disease could be avoided by incorporating healthier habits and by controlling obesity. 7 The links between diet and cancer are complex, and their participation in the pathways to carcinogenesis has not been studied in depth. 8 It has been proposed that before cancer become evident clinically it is established through well-defined sequential stages: multifocal atrophic gastritis (loss of gastric glands) → complete intestinal metaplasia → incomplete intestinal metaplasia → low-grade dysplasia and, finally, invasive cancer. Each of these stages implies a chronological, but not definitive, predisposition to the appearance of gastric cancer. For this reason they have been called precursor lesions of gastric malignancy. In 2012, their prevalence in Pasto was 38.6%. 3,4
The results of this study have shown that people who consume cereals and fats in excess of the amounts recommended in the food guides for Colombia are more likely to present precursors of gastric malignancy than are those people who consume amounts in accordance with recommended daily quantities for these two food groups. It has been estimated that more than 60% of the chemical composition of each 100 grams of the edible portion of various cereal grains corresponds to carbohydrates. A healthy and balanced diet has between 55% and 66% of these biomolecules. 9 However, consumption of larger quantities seems to be associated with alterations in glucose metabolism, hyperinsulinemia, insulin resistance, changes in the regulation of insulin growth factors (IGF Types 1 and 2), modification of the metabolism of sexual hormones, chronic inflammation, changes in the production of adipokines and vascular growth factors by adipose tissue, oxidative stress and alterations in immune function. Together, all of these factors can cause cellular events that trigger the genesis of cancer including gastric cancer. 9,10
Recently, it has been proven that diets that are high in carbohydrate content can contribute up to 72% of daily energy intake and stimulate the synthesis of IGF-1 by the unusual increase in the concentration of insulin in plasma. This particular event may be responsible for a mutagenic effect and promotion of a cascade of reactions resulting in development of a tumor. Hyperinsulinemia may be the source of the association between gastric mucosal lesions and the high energy intake. 11 A study conducted in southern India has determined that frequent and regular consumption of carbohydrates increases the risk of gastric cancer. In that study, 44% of the cases had high levels of consumption, compared to 30% in the control group. 12
A study of a population in northern Italy has determined that consumption of refined grains used for the production of white bread, pasta and rice significantly increases the risk of gastric cancer. That study concluded that there is a direct relationship between a high glycemic index and the risk of developing this type of cancer (OR 2.5, 95% CI: 1.3-4.9). 13 The explanation that prevails in these two investigations suggests that these foods promote insulin production. IGF-1 activation occurs, proliferation is stimulated, cell growth factors inhibit apoptosis and that promotes tumor cell survival. 12,13 Another study, conducted in Nariño department in Colombia, explored associations between selenium concentration and gastric cancer. It established that the consumption of cooked potatoes 6 to 7 times a week is a risk factor that can trigger gastric cancer in men. 14 Similarly, other research has found that beans, cubios (Andean tubers), turnips and potatoes, which are very common and easily acquired foods in the Andean zone of Colombia, contain large amounts of complex starches that are abrasive and difficult to digest and which may cause irritation of the gastric mucosa. These can subsequently result in formation of precursor lesions of malignancy. 15 Martínez et al. have reported that a “bread and egg” diet was positively associated with chronic atrophic gastritis (OR 2.69, 95% CI: 1.2-6.08) and metaplasia (OR 4.15, 95% CI: 1.79-9.66). 16
On the other hand, it has been found that there is a relationship between risk of cancer and total fat and saturated fatty acids in the diet. That research has found that those who consume more than 8.5 interchangeable portions of fat per day are more likely to develop gastric lesions than are those who consume fats within the recommended ranges of dietary guidelines. This may be due to the fact that lipids directly affect some cellular functions among which are the fluidity or homeostasis of the cell membrane, the metabolism of prostaglandins, and the synthesis of peroxide radicals. A diet in which fats predominate can produce changes in hormone receptors, abnormal cell growth and changes at the intracellular level. In addition, it has been reported that frequent fat consumption can cause changes in the composition of bile and initiate activation of substances in the diet which have carcinogenic capacity 17,18. In the Zhoushan Islands of China, research has found that consumption of large amounts of saturated fat, especially by men, increases the risk of gastric cancer (OR 3.24, 95% CI: 1.11-9.49). 19 Similarly, a that study found a positive association between total fat consumption and the risk of gastric cancer (OR 1.33, 95% CI: 1.12-1.57)20. Another very recent report has tried to determine an association between macronutrient intake and the risk of gastric cancer in a North American population. It found that total fat consumption (OR 1.58, 95% CI: 1.13-2.20), saturated fat consumption (OR 1.86, 95% CI: 1.37-2.52) and cholesterol (OR 1.75, 95% CI: 1.36-2.25) were significantly associated with the risk of gastric cancer. 21
Currently, research has focused on determining interactions between dietary factors and development of gastric cancer as well as the possibility that a relationship between diet, cancer etiology and cancer can be identified. The conclusion of most studies is that a diet rich in fruit and vegetables reduces the risk of developing gastric cancer22. Riboli and Norat found that for every 100 g of increased vegetable intake, the estimated relative risk was 0.81 (95% CI: 0.75-0.87). Fruit consumption, it was 0.74 (95% CI: 0.69-0.81). These figures indicate protective effects 23. Other research has concluded that the consumption of fruit (OR 0.72, 95% CI: 0.65-0.80) and vegetables (OR 0.75, 95% CI: 0, 60-0.95) have inverse associations with the appearance of gastric cancer20.
A metaanalysis of diet and gastric cancer conducted in 2007 at the Faculty of Medicine of the National University of Cheju, Korea included six hospital based case-control studies, six community-based case-control studies, and two cohort studies. It found that there is a 28% reduction in the risk of gastric cancer associated with a high levels of citrus fruit consumption (OR 0.72, 95% CI: 0.64-0.81) 24. Our study determined that the adjusted prevalence ratio for vegetable consumption was 0.67 (95% CI: 0.45-0.99) and that the adjusted prevalence ratio for fruit consumption was 0.77 (95% CI: 0.53-1.13). However, we found that only regular consumption more than two interchangeable portions of vegetables was associated with decreased risk of precursor lesions of gastric malignancy.
Our results establish that consumption of legumes, meat, dairy products and sugar are not significantly associated with precursor lesions of malignancy. However, another study to determine associations between diet and gastric carcinogenesis found that consuming processed meats was positively associated with chronic multifocal atrophic gastritis (OR 3.61, 95% CI 1.46-8.92) and gastric cancer (OR 3.10, 95% CI 0.97-9.8) but found that consumption of legumes has an inverse relationship with gastric cancer (OR 0.25, 95% CI: 0.08-0.81).
It has been found that those who use a salt shaker have a statistically significant risk of developing precursor lesions of gastric malignancy. Salt consumption has been evaluated in different ways, including study of the consumption of salty foods, study of incorporation of salt into meals after cooking, and using biochemical tests to measure the excretion of sodium in the urine. According to a group of experts from the World Cancer Research Fund (WCRF), there is a consensus about a dose dependent relationship between salt intake and the incidence of gastric cancer. It has been reported that an increase of about 8% in total salt intake (g/day) increases the risk of developing gastric lesions25.
Our study found no statistical associations between cigarette smoking or consumption of alcohol with development of gastric lesions. However, smoking is an established cause of gastric cancer, but it seems to act as a moderate risk factor in this case unlike for other cancers associated with tobacco use. A meta-analysis by Ladeiras et al. which included only prospective studies has estimated that the risk of gastric cancer for men who smoke is 1.62 (95%, CI: 1.50-1.75) and that for women who smoke it is 1.20 (95% CI: 1.01-1.43)26.
Most epidemiological studies indicate a relationship between certain dietary habits and the development of gastric cancer. Some habits increase risks while others provide protective effects. However, there are few studies of diet and precursor lesions of gastric malignancy. For this reason, our study has aimed at providing more information about the behavior of dietary habits in relation to the stages prior to the onset of gastric cancer as detected by histopathological studies. Our frequency of consumption questionnaire did not allow estimation of any exact amounts of food or nutrients consumer, and the information provided depended on the memories of respondents. The questionnaire was not validated for the population of the department of Nariño, but the food list was adjusted according to the dietary habits of the region. Nevertheless, the advantages of the questionnaire selected lies in the fact that information about eating habits could be obtained, and the usefulness of the food consumption frequency questionnaires for epidemiological studies that relate diet to disease or risk factors has been recognized.
The sample was selected from patients who had gastric symptoms and underwent digestive endoscopy during appointments with specialists. For this reason, the prevalence of precursor lesions of malignancy could have been overestimated with respect to the prevalence in the general population. The information bias was controlled by applying the questionnaire before performing the digestive endoscopy and having the final result of the presence or absence of precursor lesions of malignancy through the histopathological study. On the other hand, information losses were minimal.
Referencias
1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA: Cancer Journal for Clinicians. 2015;65(2):87-108. Doi: https://doi.org/10.3322/caac.21262 [ Links ]
2. Bedoya UA, Sansón GF, Yépez Fuertes VY, et al. Prevalencia y severidad de las lesiones precursoras de malignidad en una área de alto riesgo de cáncer gástrico. Pasto 2012. Revista Colombiana de Gastroenterologia. 2012;27:275-81. [ Links ]
3. González CA, Agudo A. Carcinogenesis, prevention and early detection of gastric cancer: where we are and where we should go. Int J Cancer. 2012;130(4):745-53. Doi: https://doi.org/10.1002/ijc.26430 [ Links ]
4. Piazuelo MB, Epplein M, Correa P. Gastric cancer: an infectious disease. Infect Dis Clin North Am. 2010;24(4):853-69. Doi: https://doi.org/10.1016/j.idc.2010.07.010 [ Links ]
5. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process. First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735-40. [ Links ]
6. Anand P, Kunnumakara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097-116. Doi: https://doi.org/10.1007/s11095-008-9661-9 [ Links ]
7. Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1191-308. Doi: https://doi.org/10.1093/jnci/66.6.1192 [ Links ]
8. Who J, Consultation FE. Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 2003;916(i-viii). [ Links ]
9. Pérez-Guisado J. Hidratos de carbono, metabolismo de la glucosa y cáncer. Endocrinología y Nutrición. 2006;53(4):252-5. Doi: https://doi.org/10.1016/S1575-0922(06)71099-3 [ Links ]
10. Florini JR, Ewton DZ, Magri KA. Hormones, growth factors, and myogenic differentiation. Annu Rev Physiol. 1991;53:201-16. Doi: https://doi.org/10.1146/annurev.ph.53.030191.001221 [ Links ]
11. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11):3109S-20S. [ Links ]
12. Laroiya I, Pankaja SS, Mittal S, et al. A study of Helicobacter pylori infection, dietary pattern and habits in patients with gastric cancer in South India. Asian Pacific Journal of Tropical Disease. 2012;2(1):24-6. Doi: https://doi.org/10.1016/S2222-1808(12)60006-1 [ Links ]
13. Bertuccio P, Praud D, Chatenoud L, et al. Dietary glycemic load and gastric cancer risk in Italy. Br J Cancer. 2009;100(3):558-61. Doi: https://doi.org/10.1038/sj.bjc.6604894 [ Links ]
14. Camargo MC, Burk RF, Bravo LE, et al. Plasma selenium measurements in subjects from areas with contrasting gastric cancer risks in Colombia. Archives of Medical Research. 2008;39(4):443-51. Doi: https://doi.org/10.1016/j.arcmed.2007.12.004 [ Links ]
15. Rodríguez A, Alvarado J, Sandler R, et al. Asociación entre infección por Helicobacter pylori y cáncer gástrico en Colombia. Acta Med Col. 2000;25:112-6. [ Links ]
16. Martínez T, Hernández G, Rojas C. La dieta y su asociación con lesiones preneoplásicas y cáncer gástrico en una zona de alto riesgo para cáncer gástrico en Colombia I, 2000-2006. Rev Colomb Cancerol. 2008;12:74-88. [ Links ]
17. van den Brandt PA, Goldbohm RA. Nutrition in the prevention of gastrointestinal cancer. Best Pract Res Clin Gastroenterol. 2006;20(3):589-603. Doi: https://doi.org/10.1016/j.bpg.2006.04.001 [ Links ]
18. Pierart C, Rozowsky J. Papel de la nutrición en la prevención del cáncer gastrointestinal. Revista Chilena de Nutrición. 2006;33(1):8-13. Doi: https://doi.org/10.4067/S0717-75182006000100001 [ Links ]
19. Qiu JL, Chen K, Zheng JN, et al. Nutritional factors and gastric cancer in Zhoushan Islands, China. World J Gastroenterol. 2005;11(28):4311-6. Doi: https://doi.org/10.3748/wjg.v11.i28.4311 [ Links ]
20. Pakseresht M, Forman D, Malekzadeh R, et al. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control. 2011;22(5):725-36. Doi: https://doi.org/10.1007/s10552-011-9744-5 [ Links ]
21. Hu J, La Vecchia C, Negri E, et al. Macronutrient intake and stomach cancer. Cancer Causes Control . 2015;26(6):839-47. Doi: https://doi.org/10.1007/s10552-015-0557-9 [ Links ]
22. Nutritional aspects of the development of cancer. Report of the Working Group on Diet and Cancer of the Committee on Medical Aspects of Food and Nutrition Policy. Rep Health Soc Subj. 1998;48:1-274. [ Links ]
23. Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(3):559S-69S. [ Links ]
24. Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and stomach cancer risk: a quantitative systematic review. Gastric Cancer. 2008;11(1):23-32. Doi: https://doi.org/10.1007/s10120-007-0447-2 [ Links ]
25. De Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. The Lancet Oncology. 2012;13(6):607-15. Doi: https://doi.org/10.1016/S1470-2045(12)70137-7 [ Links ]
26. Ladeiras-Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control . 2008;19(7):689-701. Doi: https://doi.org/10.1007/s10552-008-9132-y [ Links ]
Received: October 27, 2016; Accepted: July 28, 2017











 text in
text in