INTRODUCTION
During the last 40 years, pulmonary hypertension (PH) has been reported in genetically susceptible broilers exposed to hypobaric hypoxia 1-3.
The endothelium of pulmonary arterial branches releases vasoconstrictor and vasodilator molecules, which, in physiological conditions should be kept in an adequate balance. Under prolonged hypoxic conditions, transcription molecules such as the hypoxia induced factor 1 (HIF-1), induce liberation of vasoconstrictors such as endothelin-1 (ET-1). When this compensatory mechanism is sustained as to increase pulmonary arterial blood flow and overpass physiological levels, PH occurs 4,5. Also, nitric oxide synthase sequester can develop, thus inhibiting vasodilatation due to decrease in nitric oxide 6. This endothelial dysfunction, vasoconstrictors predominate and vascular remodeling ensues, which entails thickening of the middle muscle and the adventitia layers in arterioles 5. ET-1 appears as the most potent vasoconstrictor in the pulmonary vasculature, and the use of ET-1 inhibitors of correspondent receptors, like bosentan and intestinal vasoactive peptide to control PH has been proposed 7,8.
Given that there are diverse molecular systems which act as vasoconstrictors, it is desirable to define the degree of ET-1 participation in the pulmonary vascular response to hypoxia, in a view to contribute in the knowledge of an indicator of adaptation to hypoxia, and support future pharmacological studies which pursue the control of PH.
In a previous study a greater ET-1 mRNA expression was encountered in hypoxic pulmonary hypertensive chickens (PHC) as compared to non-hypertensive ones (NPHC) 9, but the knowledge of protein expression and correspondent sites is not defined as yet.
The aim of the present work was to quantitate ET-1 expression in the muscle layer of pulmonary arterioles of PHC and NPH ones, maintained under normoxia and hypobaric hypoxia.
MATERIALS AND METHODS
Animals and studies locations. Five hundred commercial broilers were chosen from one commercial incubating industry located at 300m above sea level (masl). One group of animals (145 chickens) were maintained at 2638 masl in Bogotá, Colombia, with a barometric pressure of 560 Hg mm, oxygen partial pressure, 117 Hg mm, and the other group were raised at 300 masl (Nilo, Colombia) under relative normoxia: barometric pressure, 740 Hg mm and oxygen partial pressure, 155 Hg mm. All animals were subjected to standard feeding and health procedures and temperature control was assessed, as to avoid obtaining readings below 20°C.
Sampling and analytical procedures. Thirty three-old chickens were slaughtered by cervical dislocation, as recommended and approved by the institutional ethical committee. PH occurrence was established by calculating the cardiac index mass (CI), obtained by calculating the total cardiac ventricular mass: right ventricular weight 10). According to previous results, PHC were considered when their CI value was above 25. Below that, animals were considered NPHC 10,11. Considering that three groups were obtained, as follows: 1) PHC raised at 2638 masl. 2) NPHC maintained at 2638 masl. 3. NPHC located at 300 masl.
The apical part of the left lung from every bird was taken, was fixed in formalin (pH 7,34) and processed with a standard method of paraffin imbibition and H & E staining. Immunohistochemical procedures were carried out using a Super Sensitive®* Polymer-HRP Detection System® (Biogenex, Fremont, USA) for protein ET-1, dilution 1:250 (Mouse monoclonal anti-endothelin 1, Thermo scientific MA3-005®, USA). Routine recommended procedures for negative and positive controls were carried out.
Four chickens per group (PHC and NPHC) were chosen for quantitative evaluation of ET-1 protein expression, In the lung of each animal, 10 arterioles with 2 to 8 smooth muscle layers were studied under the light microscope. The Image pro-plus 7 software was employed as to calculate the smooth muscle layers number with or without ET-1 protein expression, and the total area, in square micrometers, occupied by the muscle layer as well as the area with positive immune staining in the muscle layer.
Experimental design. A completely randomized model was used, and expected homogeneity of experimental material was fulfilled. The experimental error was a random independent variable with a normal distribution, zero median and variances homogeneity. When the latter was not found, corrective procedures were employed: the Welch´s and Scheffe´s tests. Likewise, Pearson correlative tests as to establish the possible association among quantitative values 12. To declare statistical differences, significance level had to be p<0.01. Data analysis was brought about with IBM® SPSS® predictive analytics software and solutions, version 17.
RESULTS
In the hypoxia exposed chickens 53% of them developed PH, and no cases of the disease were registered in the group of broilers resident in a relative normoxic environment.
Engrossment of the middle muscle layer in pulmonary arterioles (results not shown) of PHC. ET-1 expression was encountered in the endothelium, muscle cells and adventitia of blood vessels (Figure 1). Percentage of both, positive cells and area of the middle muscular layer showing expression of ET-1 was higher in animals resident at high altitude than in those placed at 300 masl (p<0.001; Figure 2). A direct association of the 2 abovementioned variables was found (r=0.954; p<0.000).
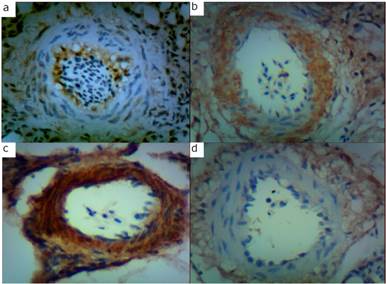
Figure 1 a: ET-1 protein expression in pulmonary arterioles of a chicken: a, maintained under relative normoxia, b: exposed to hypobaric hypoxia (non-pulmonary hypertensive), c: pulmonary hypertensive, d: maintained under hypobaric hypoxia (non-pulmonary hypertensive).
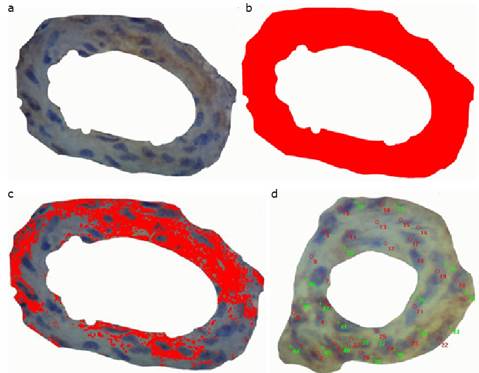
Figure 2 a, Image obtained of the middle muscle layer of an arteriole, before ET-1 expression measurements; b, image obtained of the area occupied by the middle muscle layer of an arteriole; c, results of the area (µm2) obtained after measurement; d, results obtained after counting of cells immunohistochemically expressing ET-1 protein
In figure 3 are the values obtained for ET-1 expression, which was higher in PHC placed at high altitude than in NPHC maintained at the same sea level. The latter showed higher expression than those maintained at 300 masl (p<0.001). PHC values of ET-1 showed a direct association with CI (r=0.83; p<0.001).
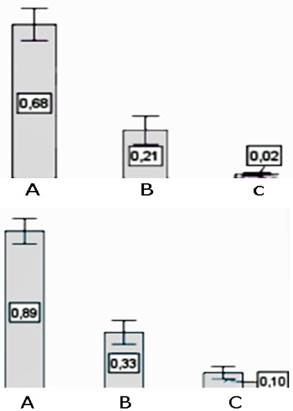
Figure 3 Percentage of the number cells expressing ET-1 protein (left side bars), and area (right side bars)occupied by them in the middle muscle layer of arterioles in: A, pulmonary hypertensive birds (subjected to hypoxia), B, non-pulmonary hypertensive birds subjected to hypoxia, and C, non-pulmonary hypertensive birds not subjected to hypoxia.
DISCUSSION
The present work corroborates the high mortality rates previously reported for the same strain of chickens under similar environmental conditions 3,11,13. It is clear that given that temperature was controlled, PH cannot be attributed to low temperature in the present study 14,15.
Hypoxia stimulates endothelial function. In susceptible individuals, augmentation of pulmonary arterial pressure results in PH and ET-1 secretion above the upper physiological limits. ET-1 migrates to muscle cells, where it enhances Ca2+ -calmodulin way and protein kinases; muscle contraction ensues and the remodeling process takes place due to a complex molecular framework 16,17. Although the mechanism of action of ET-1 in the pulmonary vessels is controversial, there is evidence that this mechanism has enough evidential support
Similar findings were reported in human pulmonary arteries of pulmonary hypertensive individuals 18.
Present results complement a previous study in which mRNA of ET-1 expression was higher in PHC than in NPHC 9,19.
The high correlation between ET-1 protein expression and CI, once more reinforces the idea that ET-1 is a fundamental molecule in the pathogenesis of PH, given its potent vasoconstrictor effect.
Several molecules, among others, have been studied as implicated in PH pathogenesis. Thus, serotonin 20, ETA and ETB (ET-1 receptors; 9,19,21), nitric oxide synthase 22-24, connective tissue growth factor and adrenomedullin 19. Given that studied molecules as key factors in PH pathogenesis are either vasoconstrictors or vasodilators of the pulmonary vasculature, they should be acting in harmony under physiological conditions and any disruption in their balance should result in the development of PH, as presently demonstrated although solely referred to ET-1 protein expression. It should be taken into account that alternative mechanism might exist for the development of PH which have been proposed, although are not to be brought into discussion at present.
The ET-1 receptors expression in the muscle layer of arterioles reinforced the involvement of ET-1 in the pathogenesis of PH 25 ,26)
The quantitative and immunohistochemical comparative findings of the present work appear to be the first ones in PHC and NPHC broilers maintained under controlled temperature conditions and subjected to natural conditions of hypobaric hypoxia and relative normoxia.














