Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.2 Bogotá abr./jun. 2013
Hepatopathology for Gastroenterologists and Hepatologists
Rocío del Pilar López Panqueva, MD. (1)
(1) Pathology Section Chief at the Hospital Universitario Fundación Santa Fe de Bogotá, Professor in the Faculty of Medicine at the Universidad de Los Andes in Bogota, Colombia. Mail: rocio.lopez@fsfb.org.co
Received: 08-05-13 Accepted: 27-05-13
Abstract
To understand the mechanisms of liver disease it is essential to link anatomical and histological information with the pathophysiological processes that occur in each disease. This first article in a series develops the basics of histopathological diagnosis.
Keywords
Liver, biopsy, pathology, diagnosis, histopathology.
The daily practice of clinical hepatology requires an extensive knowledge of the anatomy and physiology of the liver as well as the pathophysiological mechanisms which have morphological expressions that are not always well understood by the clinician.
The aim of this series is to achieve an easy understanding of histopathological findings observed in the most common diseases in clinical practice for their proper clinicopathological correlation.
1. General histopathological diagnosis
2. Useful terminology in the interpretation of the histopathological findings
3. Pathology of chronic hepatitis
4. Fatty Liver Disease
5. Diagnostic approach to cholestatic diseases
6. Drug toxicity
7. Frequent hepatic neoplasms, their differential diagnosis and utility of immunohistochemical and molecular studies.
8. Pathology of liver transplants
9. Diagnostic algorithms for liver pathology based on patterns of liver damage
10. Are liver biopsies useful? A pathologist's view.
GENERAL HISTOPATHOLOGICAL DIAGNOSIS
We begin by recalling the microstructure of the liver in which the microscopic organization is directly related to the blood supply and biliary drainage. Traditionally, we know it is arranged in hexagonal lobules, each of which has a central vein with portal triads at the ends (Figure 1). Blood flow enters through small branches of the portal vein and the hepatic artery resulting in the sinusoids near the portal tracts in the periportal area. This area, called zone 1, is rich in nutrients and oxygen. Zone 3, called the central zone or the centrilobular zone, receives a poorly oxygenated blood flow (Figure 2). The space between Zone 1 and Zone 3 is called Zone 2. This distribution is three dimensional in the hepatic acini and is considered more physiological. This explains why each of these areas has increased susceptibility to one or another type of pathology, especially the acinar areas but not necessarily the lobules, which are two-dimensional structures (1).
Hepatocytes constitute 80% of the hepatic cell population. They are arranged in cords radially distributed in the portal areas around the central vein and are separated by sinusoidal spaces (Figure 3). These spaces are lined by endothelial cells with structural and phenotypical characteristics which are different from any other vascular endothelial cells. The cells are fenestrated (lacking a basal membrane) which allows plasma and its components to be in direct contact with the hepatocytes in the subendothelial space of Disse. These cells do not express factor VIII nor do they contain typical vascular endothelia molecules such as E-selectin, CD31 or CD34 (2). They are more numerous and larger in the more distal sinusoid or perivenular areas. The fenestrae are extremely labile structures of varying diameter according to responses to endogenous mediators such as serotonin or exogenous mediators such as alcohol.
Liver macrophages or Kupffer cells which are part of the mononuclear phagocyte system constitute approximately 4% of the cell population present in the sinusoidal space. Their importance lies in their roles as part of the defense system and in the pathogenesis of various liver diseases. They are more numerous in the periportal sinusoidal spaces and migrate to injured areas (Figures 4 and 5).
Stellate or Ito cells store Vitamin A in their cytoplasm and synthesize proinflammatory cytokines and extracellular matrix proteins which mainly constitute the structural frame of the liver (3). Their role in normal liver biology is very important: by interacting very closely with the fenestrae of endothelial cells and hepatocytes in the space of Disse they help maintain differentiation, growth and functioning with the aid of surface integrin and receptors on the surface of hepatocytes. Laminin, collagen IV and heparan sulfate are found in greater amounts in the periportal area, while fibronectin, collagen III, and dermatan sulfate are abundant in zone 3 (4). Under normal conditions these cells are quiescent but can be activated by hepatocellular injury and transformed into cells that resemble myofibroblasts. They can even phenotypically express smooth muscle actin which then constitutes the major source of collagen under abnormal conditions. The cytokines secreted by the cells of Kupffer together with the inflammatory cells cause activation of stellate cells so that they can divide and perpetuate the synthesis of proinflammatory cytokines, particularly integrins. This produces an abundant extracellular matrix. Fibrosis is the tissue response to a persistent injury that promotes accelerated generation of this extracellular matrix (5, 6).
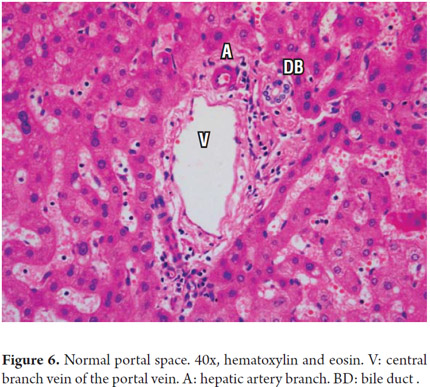
Portal areas have a supporting structure of connective tissue in which the interlobular bile duct, a branch of the portal vein and a branch of the hepatic artery can be found. The artery and the duct have approximately the same diameter (Figure 6). The ring of hepatocytes located immediately around the portal spaces is called the periportal limiting plate. This space is virtually devoid of basement membrane and is an area of vital importance in necroinflammatory diseases (Figure 7).
The cholangiocytes delineate the intra-and extrahepatic biliary systems and change the composition of bile during its transit through the bile ducts. The bile canaliculi form a mesh around each hepatocyte which drains near the interface portal into the canals of Hering. In a normal liver the canals of Hering (intrahepatic bile ductules) are inconspicuous. Measuring less than 15 microns in diameter, they are located in the periphery of the portal areas around the hepatic duct junction. They have two phenotypes and represent the physiological union between the biliary tree and the hepatocyte canalicular system extending into the hepatic lobule. They help channel the bile flow into the intestine and have absorptive and secretory functions involved in bile production (40% of total bile production is ductal). They also play an important role in regeneration and repair through proliferation cholangioles (Figure 8). The Hering channels drain the bile ductules (cholangioles) which are coated by 3-6 cholangiocytes. In the portal tracts they constitute the interlobular bile ducts that join together to form septal ducts which connect to the larger intra-and extrahepatic biliary ducts. Diameters of these ducts progressively increase while their epithelial lining changes from cubical to cylindrical (Figure 9) (7).
For optimum liver biopsy performance and results, the clinician must understand the whys and hows of the pathologist's diagnostic approach and must have a very good understanding of the meaning of the terminology used.
The pathologist should always use a systematic approach for evaluation of a biopsy, even though her or his techniques will vary according to experience. Some basic principles help interpretation and avoidance of errors.
The ideal way to start a biopsy study is with observations of all fragments, routinely stained with hematoxylin and eosin, at the lowest power of the microscope (2x or 4x) to identify the presence of focal lesions and whether or not the architecture is preserved. Identifying terminal hepatic veins, the central vein and the liver tissue surrounding it are the keys for identifying a large number of entities that compromise the perivenular area including cholestatic liver disease, alcoholic hepatitis, and fatty liver disease. The next step is to move to the portal spaces and identify their structures. This is followed by corroborating the existence of portal ducts and normal or abnormal vascular structures. It is important to observe the presence or absence of inflammation, inflammatory cell types, changes in portal collagen, alterations of bile ducts, damage to the limiting plate, and alterations of parenchymal lobules. No individual observations can be interpreted in isolation, instead all observations must be analyzed in conjunction with a complete medical history and the special techniques needed to complement the analysis of a liver biopsy.
Proper fixation of tissues is the most important pre-analytical factor for any pathological study since these studies depend on morphological preservation and increasingly depend on nuclear proteins which are necessary for molecular studies.
Routine fixation of samples should be done with 10% neutral buffered Formalin. A biopsy taken with a Tru-Cut needle requires between 2 and 4 hours of fixation. Wedge biopsies and resections require longer fixation times which can be calculated at 1 hour for each mm of tissue. Fresh tissues are needed for microbiological cultures and for biochemical analysis in metabolic diseases. Frozen tissue cuts, preserved in liquid nitrogen or at -70 ° C, are indicated for special histochemical or immunofluorescence techniques such as identification of neutral fats or microvesicular steatosis, and for evaluation of porphyrins and Vitamin A. For ultrastructural studies a small piece of no more than 5 mm should be fixed in 3% glutaraldehyde. These studies are somewhat restricted to the study of innate metabolic problems and identification of some viral infections. (1, 8)
HISTOCHEMICAL TECHNIQUES
One of the main objectives of a liver biopsy is to determine the presence and severity of fibrosis. Colorations for connective tissue are most frequently used: Masson trichrome uses a blue dye on type I collagen and reticulum uses a black dye on type III collagen. The extracellular matrix is present in normal collagen in the portal tracts (Figure 10) and on the walls of the large hepatic veins. It is most frequently used for evaluation and measurement of fibrosis. The reticle typically delineates hepatocyte trabeculae (Figure 11) which can be very useful to demonstrate collapse, confluent necrosis, atrophy and loss of trabecular reticular pattern in tumoral lesions such as hepato-carcinoma (Figure 12). Nevertheless, because it is not possible to differentiate scarred areas of a collapse, these are considered complementary studies (9, 10).
Prussian blue staining for iron allows detection of hemosiderin. The blue deposit corresponds to iron deposits. Both the quantity and location are evaluated to define potential etiologies (Figure 13). This coloration also accentuates other deposits such as bilirubin which has a green hue and lipofuscin which is golden yellow or brown.
Copper has an orange hue in cytoplasmic granules when detected through the use of rubeanic acid or rhodamine (Figure 14). Copper can only be detected in Buffered formalin-fixed tissues since no other fixative allows for its identification. The determination of these deposits is important for diagnosis of Wilson's disease and in chronic cholestatic diseases. Copper-binding protein can be demonstrated with orcein, aldehyde fuschine or Victoria blue. Victoria blue also allows identification of binding protein deposits in cholestatic liver disease (Figure 15) and ground-glass inclusions in hepatitis B which correspond to the surface antigen.
Periodic acid-Schiff (PAS) staining with and without diastase digestion (PAS-D) plays an important role in the diagnosis of liver disease. Liposfuscin and PAS positive keloid materials deposited in in Kupffer cells and portal macrophages appear in recent hepatocellular injury foci. They help to demonstrate the presence of basal membranesin cholangioles and bile ducts. Periportal cytoplasmic globules which indicate α-1 antitrypsin deficiency are PAS positive (Figure 16). Although this coloration is ideal for demonstrating the presence of glycogen, formalin fixative allows cells to lose glycogen. Consequently, if it is necessary to demonstrate the presence of glycogen, the tissue must be fixed in alcohol.
In addition to these routine studies it is possible to perform histochemical tests for amyloid, fibrin and microorganisms such as bacteria, fungi (Figure 17) and for any other thing that is warranted (10).
IMMUNOHISTOCHEMICAL TECHNIQUES
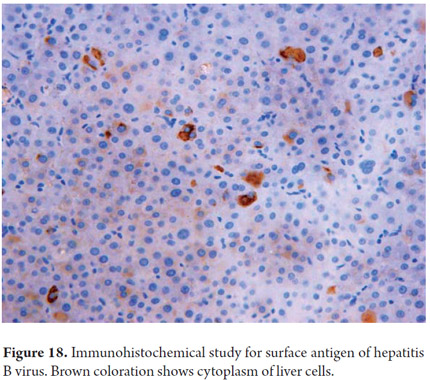
These are now routine procedures in pathology laboratories. Currently one of the major roles of immunohistochemistry is phenotyping primary and metastatic hepatic malignancies (11, 12). Immunohistochemistry has many other used. They include sub-typing hepatic adenoma based on the immunohistochemical expression of their mutations and for differential diagnosis between well-differentiated Hepatocellular carcinoma and focal nodular hyperplasia (13, 14). Another use is demonstration of the presence of bile ducts especially in diseases which reduce the number of ducts. Still another use is confirmation of the presence of microorganisms or viral inclusions of α1 antitrypsin globules, surface antigen (Figure 18) and hepatitis B virus (11, 12). Table 1 exemplifies some of the most commonly used antibodies in daily practice.
MOLECULAR TECHNIQUES
There are two types of molecular techniques: in situ" (ISH) and "ex situ." In situ techniques include chromogenic in situ hybridization (CISH) and fluorescent in situ hybridization (FISH). Ex situ techniques require preliminary extraction of the target molecule to obtain cellular components with enough DNA or RNA for amplification using polymerase chain reaction. Proper fixation is essential as the quality and integrity of the nucleic acids depends on it. Since extraction efficiency is inversely proportional to the time of fixation, the fixation process must be properly standardized. In situ techniques are especially used for detection of Epstein Barr virus (EBV RNA), for diagnosis of post transplant lymphoproliferative disorders, for diagnosing CMV and other sub-proteins, and for diagnosing tumoral diseases. Molecular tests include clonality testing, mutation analysis, and comparative genomic hybridization (1, 14).
REFERENCES
1. Burt A, Portmann B, Ferrel L. MacSween's Pathology of the Liver. Sixth edition. Churchill Livingstone Elsevier 2012. [ Links ]
2. Scoazec JY, Feldman G. In Situ phenotyping study of endotelial cells of the human hepatic sinusoid: results and functional implications. Hepatology 1991; 14: 789-97. [ Links ]
3. Asahina K. Hepatic stellate cell progenitor cells. J Gastroenterol Hepatol 2012; 27 Suppl. 2: 80-4. [ Links ]
4. Reid LM, Fiorino AS, Sigal SH, et al. Extracellular matrix gradients in the space of Disse: relevance to liver biology. Hepatology 1992, 15: 1198-1203. [ Links ]
5. Enzan H, Himeno H, et al. Sequential changes in human to cells and their relation to postnecrotic liver fibrosis in massive and submassive hepatic necrosis. Virchows Arch 1995; 426: 95-101. [ Links ]
6. Eleonora Patsenker, Felix Stickel. Role of integrins in fibrosing liver diseases. Am J Physiol Gastrointest Liver Physiol 2011; 301: G425-G434. [ Links ]
7. Strazzabosco M, Fabris L. Functional anatomy of normal bile ducts. Anat Rec 2008; 291(6): 653-60. [ Links ]
8. Bonin S, Hlubek F, Benhattar J, et al. Multicentric validation study of nucleic acids extraction from FFPE tissues. Virchows Arch 2010; 457(3): 309-317. [ Links ]
9. Feldmann G. Critical analysis of the methods used to morphologically quantify hepatic fibrosis. J Hepatol 1995; 22: 49-54. [ Links ]
10. Lefkowich JH. Special Stains in diagnostic liver pathology. Sem Diagn Pathol 2006; 23: 190-198. [ Links ]
11. Roskams T. The role of immunohistochemistry in diagnosis. Clin Liver Dis 2002; 6: 571-589 [ Links ]
12. Geller SA, Dhall D, Alsabeh R. Application of immunohistochemistry to liver and gastrointestinal neoplasms: liver, stomach, colon and pancreas. Arch Pathol Lab Med 2008; 132: 409-499. [ Links ]
13. Jessica Zucman-Rossi et al. Genotype-Phenotype Correlation in Hepatocellular Adenoma: New Classification and Relationship with HCC. Hepatology 2006; 24: 515-24. [ Links ]
14. Paulette Bioulac-Sage, et al. Hepatocellular Adenoma Subtype Classification Using Molecular Markers and Immunohistochemistry. Hepatology 2007; 46: 740-748. [ Links ]











 texto en
texto en