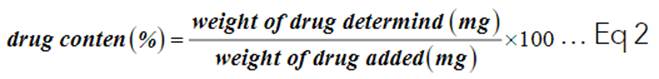INTRODUCTION
Famotidine is a crystalline powder that is white to pale yellowish-white in color. It is very slightly soluble in water, practically insoluble in alcohol, acetone, ether, and ethyl acetate, and freely soluble in dimethylformamide. Oral Famotidine bioavailability of about 40 - 45 percent is unaffected by food presence. The plasma elimination half-life is observed to be around 3 hours, extending in renal impairment 1.
Famotidine is used to treat benign gastric and duodenal ulcers, gastroesophageal reflux disease, short-term symptomatic relief of heartburn or non-ulcer dyspepsia, and the Zollinger-Ellison syndrome, blocking the effect of histamine and therefore lowering hydrochloric acid production. The usual dose is 20-40 mg, given once or twice a day. It comes as a tablet, chewable tablet, oral suspension, and injectable solution, among other pharmaceutical forms 2.
The tablet dosage form is the most commonly recommended in the healthcare context. Several brands of famotidine tablets are available in pharmacies that are considered pharmaceutically equivalent if they contain the same amount of active components in an identical dosage form and meet the same compendia standard (i.e., strength, purity, and quality). Still, they may differ in shape, packaging, additive material, and labeling requirements. Similarly, there are numerous generic famotidine tablets available in Iraqi pharmacies. The World Health Organization ( WHO) has consistently supported the use of generic brands to decrease the cost of medications, particularly for low-income groups in developing countries. However, this strategy has not produced adequate evidence supporting the substitution of one brand for another 3.
A healthcare practitioner is concerned about choosing one product among numerous generic medication products containing the same active components during therapy. The first step in determining any medicinal product’s therapeutic equivalence is its chemical and biological equivalent. As a result, monitoring authorized drugs is required to appropriately examine drugs’ quality, therapeutic efficacy, and safety for the general population. This quality control testing might be performed before and after production and at various intervals throughout a drug product’s shelf life 4.
Many post-market monitoring studies on various drug items were conducted in several developing countries, yielding essential specifications. In Iraq, for example, Saad M.M. et al. performed a quality control study on traditional Metronidazole tablets available in the Iraqi market. The results showed that nearly all the tested brands of Metronidazole tablets were adequate, having passed all of the pharmacopeial necessities for oral tablets 5.
Conversely, J.R. Medina et al. investigated the dissolution characteristics of four carbamazepine immediate-release generic products and the reference product Tegretol (brand) using the USP paddles technique and an alternative approach utilizing the flow-through cell system, USP Apparatus 4 6.
Additionally, Adegbolagun et al. evaluated the drug content of ten generic ciprofloxacin tablets. They discovered that three products had active components at levels lower than USP standards for ciprofloxacin HCl content (90-1 0 %), while the remaining met USP specifications 7.
Consequently, the objective of this study was an evaluation of four distinct famotidine 20 mg tablet products. One of which is a brand and the other three are generic, marketed in Iraq by using official and non-official standards tests according to USP specifications, attempting to emphasize whether or not all of the brands and generic are pharmaceutically equivalent. Tablet evaluation comprises hardness, friability, in-vitro dissolution study, the kinetics of drug release, and evaluating the content of active pharmaceutical ingredients.
MATERIALS AND METHODS
Materials
Four different samples of the most commonly available Famotidine 20 mg tablets in the Iraqi market were purchased from the private pharmacy, as shown in Table 1. Famotidine standard powder was a generous gift from SDI, Samra, Iraq. The other compounds were of analytical quality.
Methods
I. Characterization of Famotidine pure powder
A. Determination of λmax of Famotidine
Twenty milligrams of Famotidine were dissolved in 100 mL 0.1N HCL to prepare a 0.2 mg/mL stock solution. From this stock solution, a dilute (25ug/mL) solution was prepared and scanned by a U.V. spectrophotometer at the range of 200-400 nm 8.
Table 1 The evaluated marketed famotidine tablets
| Name of product | Sample code | Company Name; | Strength in mg | Batch number | Origin Country |
|---|---|---|---|---|---|
| Famosam® | FM-A | SDI | 20 | 1 | Iraq |
| Ulceran® | M-B | Medochemie LTD | 20 | E4k104 | Cyprus (Europe) |
| Famodar® | FM-C | Dar Al-Dawa | 20 | 1232Y | Jordan |
| Famodin® | FM-D | Sandoz | 20 | B3073 | Italy |
B. Preparation of calibration curve.
The famotidine calibration curve in 0.1N HCl (pH 1.2) was created by making a series of solutions with varying concentrations of Famotidine from a stock solution containing 0.2 mg/ml of Famotidine. These solutions were then spectrophotometrically evaluated using a U.V. spectrophotometer at the wavelength of maximum absorbance. Each sample’s absorbance was measured and plotted against its concentration 9.
II- Evaluation tests for the selected Famotidine products:
1. Hardness:
A tablet’s crushing strength was determined using a Monsanto hardness tester. Five pills from each brand and three generic products were chosen at random, and the force at which each tablet was crushed was measured in kg/cm2 (10.
2. Friability test:
Before testing, ten randomly selected tablets from each generic and brand product were weighed accurately in the friabilator chamber. The friability equipment was turned on for 100 revolutions, and the weight difference was measured by weighing the tablet after the test. The following formula (Equation (1)) was used to compute the friability % 11:
3. Drug content of tablets formulation.
Ten famotidine tablets were taken from each generic and brand product, placed in a mortar, and crushed into a fine powder. An accurately weighed powder equivalent to 20 mg of Famotidine was transferred into a 100 ml volumetric flask holding 100 ml of 0.1N HCl, and the mixture was agitated for 30 min. After that, 5 ml aliquots were withdrawn and filtered using Whitman filter paper. The filtrate was analyzed in U.V. spectrophotometrically at 266 nm 12.
Famotidine content % was calculated from Equation (2):
4. In-vitro dissolution study:
The release rate of Famotidine from generic and brand-name tablets was measured in triplicate using the USP paddle technique and the Dissolution Testing Apparatus 2. The dissolution experiment was carried out at 50 rpm using 900 mL of 0.1N HCL as the dissolution medium.
Samples were taken at predetermined intervals (e.g., 10, 20, 30, 45, 60, and 90 minutes) and filtered using Whatman filter paper. 5 ml of fresh medium was added after each 5 ml sample was taken to keep the sink condition. The filtrate was evaluated using a U.V. spectrophotometer at 266 nm., the percentage drug release is plotted versus time to calculate the release profile 13.
5. Release kinetics.
The results of in-vitro release profiles obtained for the investigated products of famotidine tablets were fitted into three models of the data treatment as follows:
Zero-order kinetics.
In its simplest form, zero-order release can be represented as by Equation (3):
Qt: the amount of drug release or dissolved in time t.
Q0: the initial amount of drug in the solution.
K0: zero-order release kinetic constant.
Data from in-vitro dissolution tests is plotted as the percent cumulative quantity of drug released against time in zero-order release kinetics 14.
First-order kinetics.
Noyes and Whitney developed the first quantitative equation for describing dissolution rate, and the integral form of the equation is represented by Equation (4) 15.
here C0 is the initial amount of drug and K is the first-order constant.
Ct: the amount of drug dissolved in time t.
Higuchi model.
Higuchi shows drug release as a diffusion mechanism based on the square root of time. The collected data were shown as percent cumulative drug release vs. square root of time (14). It can simplify the Higuchi model as Equation (5):
KH. is the Higuchi dissolution constant.
Model Independent Methods
The theories and kinetic models relating to drug release forms and drug solubility are the difference factor (f1), similarity factor (f2) 16.
where, n is the number of sample points, Rj is the dissolution value of reference at time t, Tj is the dissolution value of test at time t.
RESULTS
A. Analytical evaluations of Famotidine powder
Scanning of Famotidine.
The maximum absorbance (λ max) wavelengths for diluted (25 ug/mL) solution of Famotidine in 0.1N HCL showed only one strong absorption peak at 266 nm. The obtained results agree with the reported before 8.
Standard calibration curve of Famotidine in 0.1N HCL.
The calibration curves for Famotidine in 0.1 N HCl (pH 1.2) were created and shown in Figure 1. The curve was determined to be linear with a regression value of 0.9997, indicating that the chosen concentration of famotidine solutions follows Beers law. This calibration curve was used to calculate in-vitro drug release and drug content.
B- Quality control tests of selected product:
The results of official and unofficial quality control tests conducted on brand and three different generic famotidine tablet products sold in Iraq are summarized in Table 2, which shows the average value for tablet hardness, percent of friability, and percent of famotidine content in each sample of the product.
Figure 2 depicts the release dissolution profiles of the brand and the different generics of the available famotidine tablets tested, as measured by the percent of drug released at a particular time using the famotidine calibration curve equation (each value represents mean ± S.D., n = 3).
Table 2 Results of official and unofficial quality control tests on the three generic and brand of famotidine tablets.
| Name of product | Sample code | famotidine Content (%) Mean ± S.D. (n=3) | Friability (%) Mean ± S.D. (n=10) | Hardness (kg/cm2) Mean ± S.D. (n=5) |
|---|---|---|---|---|
| Famosam® | FM-A | 96.86 ±1.23 | 0.032 ±0.07 | 7.2 ±0.22 |
| Ulceran® | FM-B | 97.9 ± 0.97 | 0.02 ±0.051 | 14.4±0.57 |
| famodar® | FM-C | 101.1 ±1.12 | 0.021 ±0.067 | 9.9±0.332 |
| Famodin® | FM-D | 99.87 ±1.11 | 0.015 ±0.026 | 5.7±0.38 |
DISCUSSION
The tests on famotidine powder, such as spectrophotometer scans, revealed that the powder sample utilized in this study was pure, with a wavelength of 266 nm, consistent with the reported values in the USP-30 8.
The calibration curves of the standard famotidine solutions were generated, as shown in Figure 1, yielding a linear regression equation utilized to calculate the drug content of the tested tablets and the percent of drug released during the dissolution investigation.
The hardness is a valuable parameter to assess the ability of the individual tablet to withstand the mechanical stress and keep structural integrity under different conditions of processing, storage, transportation, and handling before usage. If the pills are too hard, they may not disintegrate in the required time, and if it too soft, they may break during the handling of the manufacturing or packaging process. This physical property is influenced by many factors like formulation ingredients, processing parameters, and storage conditions 17.
The tablet hardness was measured as kg/cm2 for the tested product of famotidine tablets. The result shows that the highest value was 15.7 kg/cm2 for Famotidine, the brand product, and the lowest value was 7.2 kg/cm2 for Famosam produced by SDI. As per BP and USP a force of about 4-6 kg/cm2 is the minimum requirement for a good tablet. Thus, the tablets of all the products were adequate for proper hardness. It is worth noting that the high hardness ratings for all the products may be attributable to the film coat applied to the surface, which increases mechanical strength; this finding is in line with another study conducted on coated tablets 18.
Friability tests can be used to assess tablets’ resistance to stresses such as mechanical shocks and abrasion throughout the production, packaging, and shipping processes. According to the USP standard, the tablet formulation is acceptable if the friability is less than 1% 8.
As shown in Table 2, the values of friability percent for the selected brand and the generic famotidine products chosen for this study were relatively low. This fact may be related to the coat around the tablet, which indicated that all the brands were mechanically stable and met the pharmacopeial requirements 19.
The average content of analyzed Famotidine tablets by the U.V. spectrophotometric method was found to be in the range of 96.86 % - 101.1% (Table 2). The highest amount of Famotidine was presented with generic Famodar®, which contains 101.1% of the labeled amount. In contrast, the lowest amount of famotidine content was found in the generic product, Famosam, calculated as 96.86% of the labeled amount. Consequently, branded products tagged as Famodin had a drug content of 99.87% within the acceptable range of pharmacopeial specifications. The four tested products had famotidine content within the acceptable range of USP and B.P. requirements.
Regarding the dissolution study, the in-vitro dissolution investigation provides helpful and accurate information concerning the in-vivo bioavailability of drug content. These tests record the rate and extent of drug substances released from a dosage form by registering the percent of drugs released at a specific time. The dissolution rate of the film-coated immediate-release tablet should not be less than 80% of the stated quantity in 30 minutes (20-21).
Figure 2 shows in-vitro release profiles for the investigated generic and brand famotidine products. Brand Famodin showed the highest drug release rate, whereas generic Famosam had the slowest release rate within the same time intervals. Brand Famodin had maximum drug release, almost 97% of drug content, while generic Famosam showed minimum drug release at this time, which was 79%. All the tested products reached 100% drug release after 45 minutes. After 30 minutes, a comparison of the dissolution data revealed that all the tested brands and two generic Famotidine tablets met the USP specification for the dissolution limitation and possessed good dissolution profiles.
The calculated f 1 factor for the three generics products was less than 15 for Famodar and Ulceran tablets, while it was 18 for Famosam. The f2 for Ulceran and Famodar were 45 and 49 respectively, near 50 (accepted value 50-100), while it was 40 for Famosam as mentioned in Table 4. This dissolution efficiency and the 95% confidence intervals were within ±10% only for the generic Ulceran and Famodar tablets.
It showed that brand Famodin tablets and other generic products (Ulceran and Famodar) were within the aforementioned requirements and can be considered pharmaceutically equivalent. As a result, clinicians can prescribe them interchangeably in cases of unavailability or reduce treatment costs.
Exceptional to them Famosam did not fully specify the requirements.
Regarding Drug Release Kinetics, the release mechanisms of Famotidine from selected products were evaluated by general dissolution equations, including zero order, first order, and Higuchi model. Due to different release kinetics were assumed to reflect other release mechanisms as shown in Figures 3, 4 and 5, for zero, first, and Higuchi models, respectively. The obtained results are shown in Table 3
The release pattern of Famotidine in 0.1N HCl showed higher correlation coefficients when they were fitted to the first-order model, R2 value above 0.917, describing the drug release rate relationship with drug concentration.
Higuchi’s equation plot (Figure 5) (R2 = 0.98-0.99) indicated the drug release from the matrix as a square root of time-dependent process based on non-Fickian Super Case II type diffusion 14.
Table 3 The Release Mechanism of the brand and the generic Famotidine tablets in 0.1N HCl (pH 1.2) at 37±0.5 C0
| Product name | R² value | |||
|---|---|---|---|---|
| Formula no | Zero-order | First-order | Higuchi | |
| Famosam | FM-A | 0.946 | 0.947 | 0.998 |
| Ulceran | FM-B | 0.924 | 0.935 | 0.993 |
| Famodar | FM-C | 0.936 | 0.917 | 0.995 |
| Famodin | FM-D | 0.887 | 0.978 | 0.981 |
Table 4 Difference factors (f1) and similarity factor (f2) for the generic product as compared with brand
| Generic | f1 | f2 |
|---|---|---|
| Famosam | 18 | 40 |
| Famodar | 13 | 49 |
| Ulceran | 13 | 45 |

Figure 3 Zero-order drug release kinetics of the brand and the three generics tested famotidine tablets available in Iraq

Figure 4 First-order drug release kinetics of the brand and the three generics tested famotidine tablets available in Iraq
CONCLUSION
This study aimed to compare the pharmacological characteristics and in-vitro bioavailability of three generic famotidine tablets and one brand-name famotidine tablet on the Iraqi market. Various quality-control tests were conducted to determine if these brand items are pharmaceutically comparable to generic products. The findings showed that virtually all the tested brands and generic Famotidine tablets met all the pharmacopeial standards for oral tablets. According to dissolution tests, brand Famodin and generic drugs had release profiles comparable to the innovator product. As a result, when fetching the innovator product of famotidine tablets is problematic, the tested products might be recommended to healthcare practitioners as an alternative.