INTRODUCTION
The blackberry crop (Rubus spp.) is considered an important species within the small fruit group and has been standing out in recent years for presenting good prospects for cultivation in family farming areas (Fachinello et al., 2011; Antunes et al., 2014). In Brazil, cultivation has been increasing considerably, especially in the southern and southeastern regions of the country (Schiavon et al., 2021), where the best climatic conditions are available.
The propagation of blackberries can be mainly carried out through shoots, herbaceous, semi-hardwood, and hardwood cuttings, root cuttings, and tissue culture (Dias et al., 2012; Crosa et al., 2021). According to Antunes et al. (2000), the use of stem cuttings as propagation material is recommended, which are collected from the plants during the winter pruning season.
Limitations in the vegetative propagation of species through the method of cuttings are presented by several factors, among which we can mention the lack of efficient methods for rejuvenating adult material and adequate management techniques in the propagation medium (Dias et al., 2015). Ensuring success will depend not only on the genetic potential for rooting, but also on the physiological conditions of the parent plant, the time of year, weather conditions, and hormonal balance (Campbell et al., 2021).
For blackberry, specifically, quite varied results have been observed regarding its ability to form adventitious roots from stem cuttings. However, there is a predominance of results that indicate its low capacity for adventitious rooting when it comes to stem cuttings (Picolotto et al., 2015; Hussain et al., 2017).
One method to promote and facilitate root development in the production of difficult-to-root seedlings is the exogenous application of growth regulators (Rocha et al., 2020). Therefore, aiming to increase the rooting of stem cuttings, the use of solutions of indole butyric acid (IBA), a synthetic auxin that induces root initiation, increases and standardizes the number and quality of formed roots (Han et al., 2009; Jamal et al., 2016). This is so true that when applied under an adjusted methodology for each plant, an increase in rooting has been obtained (Pigatto et al., 2018; Ada and Enrico, 2020; Ötvös et al., 2021).
Even with the application of auxins, the results obtained in blackberry propagation by stem cutting have not been very promising (Villa et al., 2003; Campagnolo and Pio, 2012; Debner et al., 2019), which points to the need to develop other propagation techniques that can assist in this process and promote better results.
In this regard, other growth regulators have been associated with the adventitious rooting process to promote propagation by stem cuttings. In this case, paclobutrazol (PBZ) stands out, which has been studied in plants with different patterns of adventitious root growth, since, based on the premise that this substance promotes inhibition of gibberellin biosynthesis (Desta and Amare, 2021) and, therefore, inhibition of leaf growth (sprouting), it would favor the formation of adventitious roots in response to the increased transport of hormones and/or assimilates towards the base of the cuttings in support of adventitious rooting (Zheng et al., 2016; Jabir et al., 2017).
These effects in promoting rooting with PBZ have been observed by Qadri et al. (2018), Bueno et al. (2021), and İşbilir et al. (2022) in promoting roots in juvenile guava cuttings (Psidium guajava L.), green blackberry stem cuttings (Rubus brasiliensis Mart.), and hardwood blackberry stem cuttings (Morus nigra L.), respectively.
For these reasons, the present study aimed to evaluate the rooting of ‘Tupy’ blackberry (Rubus spp.) stem cuttings with the application of growth regulators (IBA and PBZ) at different concentrations.
MATERIALS AND METHODS
The cuttings used in this study were obtained from ‘Tupy’ blackberry plants grown at the Canguiri Experimental Station, located in the municipality of Pinhais-PR, under the coordinates 25°23’30” S and 49°07’30” W. On August 11, 2019, branches from the middle part of the plants were collected, immediately moistened and placed in plastic bags. The branches were then transported to the greenhouse where they were prepared into 10 cm cuttings, with a bevel cut at the base and a longitudinal cut at the top. Subsequently, the cuttings were disinfected with a 0.5% sodium hypochlorite solution for 10 min and rinsed with running water for 5 min.
The treatments consisted of three hydroalcoholic concentrations (50% v/v) of IBA (500; 1,000 and 2,000 mg L-1) and three concentrations of PBZ (100, 200, and 400 mg mL-1), each for a period of 10 s, in addition to control treatments that were prepared with hydroalcoholic solutions (50% v/v) without adding IBA and PBZ.
The completely randomized experimental design was used in a 4×4 factorial scheme (0; 500; 1,000 and 2,000 mg L-1 of IBA × 0, 100, 200, and 400 mg mL-1 of PBZ) with three replicates and 10 cuttings per experimental unit, totaling 480 cuttings.
The planting of the cuttings was carried out in 114 cm3 plastic tubes containing medium-sized vermiculite as a substrate. The experiments were set up in a greenhouse with intermittent misting at 90% relative humidity (30 s every 30 min from 08:00 am to 05:00 pm and 30 s every 2 h from 05:00 pm to 08:00 am).
The evaluations were carried out 120 d after the experiment installation to obtain data on the percentage of rooted cuttings (considered rooted when they presented at least one adventitious root of 1 mm or more in length), percentage of cuttings with callus (without roots but with callus), percentage of live cuttings (those that did not root or form callus but were not yet necrotic), percentage of dead cuttings (necrotic), percentage of cuttings with sprouts, length of sprouts (measured with a digital caliper), number of roots formed per cutting, the average length of the three largest roots per cutting, and roots fresh weight.
The obtained data were subjected to analysis of variance and subsequent comparison of means by Tukey's test at a 5% significance level, for the variation factors whose effect was significant. The Shapiro-Wilk test was applied to verify the homogeneity of the data, and data transformation by
RESULTS AND DISCUSSION
The analysis of variance performed on the observations on root formation and growth of blackberry stem cuttings did not show a significant favorable effect on the treatments performed (Tab. 1), and there was no interaction between the treatments performed with IBA and PBZ, nor the effect isolated from PBZ for none of the traits evaluated. On the contrary, it manifested a significant effect with the IBA treatment, but in a negative way, harming an excellent growth of the cuttings.
Table 1 Summary of the analysis of variance of the means of the characteristics percentage of rooted cuttings (RC), sprouted cuttings (SC), dead cuttings (DC), and with callus (CC), average root length (RL), number of roots (NR), and roots fresh weight (RFW) of woody cuttings of blackberry cv. Tupy treated with indole butyric acid (IBA) and paclobutrazol (PBZ).
| FV | GL | RCa (%) | SCa (%) | DCa (%) | CCa (%) | RLb (cm) | NRb | RFWb (g) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QM | F | QM | F | QM | F | QM | F | QM | F | QM | F | QM | F | ||
| IBA | 3 | 19.99 | 9.45** | 41.19 | 15.36** | 7.54 | 15.88** | 17.98 | 9.29** | 18.26 | 6.22** | 5.42 | 7.19** | 0.81 | 5.93** |
| PBZ | 3 | 0.40 | 0.19ns | 1.18 | 0.44ns | 68.28 | 143.71** | 0.61 | 0.32ns | 2.72 | 0.93ns | 0.46 | 0.61ns | 0.13 | 0.92ns |
| IBAxPBZ | 9 | 4.01 | 1.89ns | 2.35 | 0.87ns | 19.18 | 40.38** | 3.27 | 1.69ns | 1.77 | 0.60ns | 0.98 | 1.30ns | 0.09 | 0.70ns |
| CV (%) | 65.2 | 57.4 | 8.43 | 72.49 | 111.8 | 104.9 | 121.5 | ||||||||
FV - Variation factor; GL - Degrees of freedom; QM - Mean square; F - F statistic value; CV - Coefficient of variation. **Significant at 0.01% probability; ns not significant. a Data transformed by
Based on the results obtained, in general, the use of growth hormones, either individually or in combination, had a negative effect on the formation of blackberry stem cuttings cv. Tupy. These results were similar to those found in previous studies on blackberry by Andrade et al. (2007) and Tadeu et al. (2012) when they tested IBA, and by Maia and Botelho (2008) when they separately evaluated IBA and PBZ.
Regarding PBZ, Desta and Amare (2021) found that in different studies, the application of the growth regulator at moderate concentrations stimulated the growth of adventitious roots (length and width) due to the promotion of cell expansion. In contrast, in the study by Qadri et al. (2018) using guava cuttings, low concentrations of PBZ not only stimulated optimal root growth but also increased the number of sprouts and survival, due to a greater translocation of carbohydrates in the formation of shoots. However, in this study, PBZ applications at different concentrations had no effect on blackberry cuttings, probably due to the crop's herbaceous nature and the growth regulator's toxicity.
For both the rooting percentage (Fig. 1) and sprouting percentage (Fig. 2) of blackberry stem cuttings of the Tupy cultivar, it was observed that in the absence of the IBA hormone, the highest values were obtained with 17.73 and 28.83%, respectively. On the other hand, the higher the increase in the concentration of the IBA hormone, the greater the damage, with the dose of 500 g L-1 presenting the minimum considerable damage in the evaluated variables.
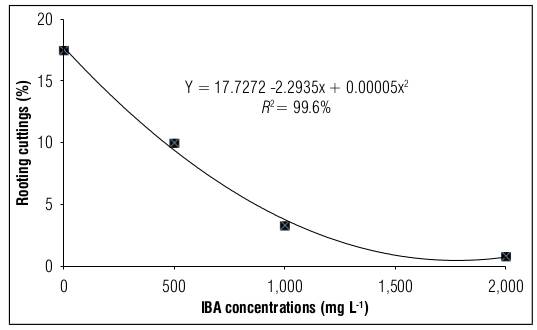
Figure 1. Rooting of blackberry stem cuttings cv. Tupy treated with different concentrations of indole butyric acid (IBA).
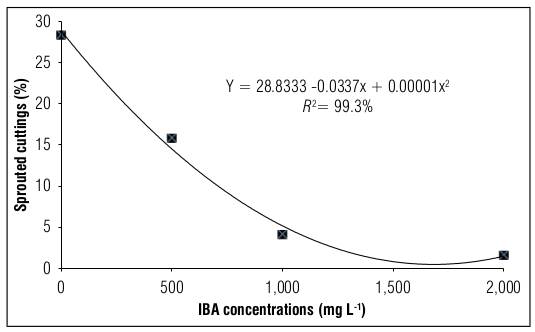
Figure 2. Sprouted blackberry stem cuttings cv. Tupy treated with different concentrations of indole butyric acid (IBA).
Hartmann et al. (2010) and Dias et al. (2011) argued that high concentrations of IBA in vegetative propagation via cutting can cause negative effects on cuttings such as imbalances in endogenous levels (especially in auxins), and phytotoxicity in rooting induction ultimately resulting in death in blackberry cuttings. This was the case in the present study and in those conducted by Campagnolo and Pio (2012), Moreira et al. (2012), Hussain et al. (2014), Tiberti et al. (2012), Ahmed et al. (2018), Villa et al. (2018) and Debner et al. (2019) in various berry crops and in different seasons, respectively.
Shoot sprouting is one of the most important characteristics in the production of quality seedlings because the presence of new shoots ensures the nutrition of future plants after the depletion of cutting reserves (Gomes and Krinski, 2016). However, in the present research, this did not happen, as the percentage of shoot sprouting was negatively affected by the presence of IBA, and the results indicated that treatment with the growth hormone is dispensable for shoot sprouting in blackberry stem cuttings. These results are in agreement with those obtained by Villa et al. (2003), Tadeu et al. (2012), Campagnolo and Pio (2012), and Tiberti et al. (2012) who reported a reduction in the shoot sprouting of blackberry and ‘boysenberry’ (hybrid obtained by crossing Rubus ursinus x Rubus idaeus) stem cuttings treated with high concentrations of IBA.
Regarding the evaluation of the percentage of dead cuttings in blackberry, the lowest value was obtained in the absence of the growth regulator (71.17%); however, in the presence of IBA, the highest value was observed (99.22%) at the dose of 1,500 g L-1 (Fig. 3). As for the percentage of callus, the highest value was obtained in the absence of the plant hormone (14.16%), and the presence of IBA hindered callus formation, even leading to zero formation at high concentrations (Fig. 4).
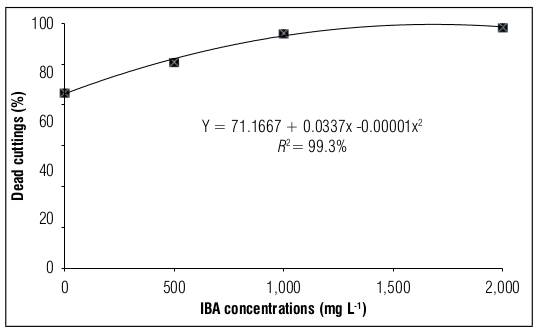
Figure 3. Dead blackberry stems cuttings cv. Tupy treated with different concentrations of indole butyric acid (IBA).
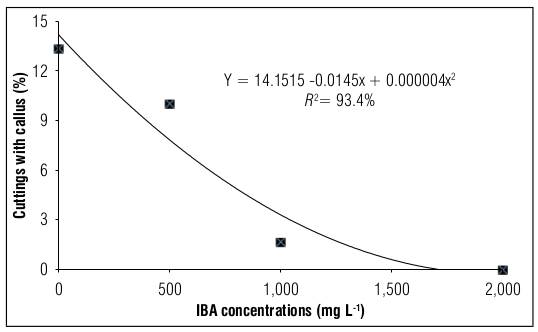
Figure 4. Blackberry stem cuttings with callus cv. Tupy treated with different concentrations of indole butyric acid (IBA).
The survival of cuttings in asexual propagation is determined by the complex interaction between environmental and internal factors (Li et al., 2009). In this study, blackberry cuttings that were propagated in the summer were not influenced by it; however, as Han et al. (2009) pointed out, the survival of cuttings is mainly determined by internal factors, where carbohydrate reserves are related to the success of higher percentages of rooting. Therefore, we could say that in the present study, the concentrations of IBA interfered with this process, gradually increasing the concentration of synthetic auxin within the tissue and in turn causing mortality of the cuttings. These results were also verified in the studies of Dias et al. (2011), Hussain et al. (2014), and Ahmed et al. (2018) when they evaluated root cuttings and mini cuttings of blackberry, respectively.
In the propagation of cuttings with difficulty in rooting, callus formation is a sign of possible future rooting (Bitencourt et al., 2010). This process is influenced by the increase in the optimal concentration of the hormone IBA, which stimulates root growth and development of the root system (Gilani et al., 2019). However, opposite results were observed in blackberry cuttings in the present study, where the percentage of callus showed similar behavior to the growth parameters already described, that is, callus formation was negatively influenced by IBA doses, as verified by Campagnolo and Pio (2012) in cuttings of the same cultivar of the present study, but which were mounted during the winter. On the other hand, in blackberry cuttings of the Xavante cultivar, no significant differences were found in callus formation when different IBA concentrations were evaluated (Maia and Botelho, 2008).
Analyzing the effect of the IBA hormone on the growth parameters of blackberry cuttings (Tab. 2), it can be observed that there was a significant difference in the concentrations of the growth regulator. The dose of 500 mg L-1 stood out among the other treatments, with the root length presenting the highest average of 12.14 cm, followed by the absence of the hormone at 8.45 cm. As for the number of roots, the aforementioned dose obtained the highest average of 3.87 compared to the absence of the hormone with 2.00. For the roots fresh weight, the same dose was recorded at 0.58 g, followed by the absence of the hormone at 0.36 g, respectively.
Table 2 Root length (RL), number of roots (NR), and roots fresh weight (RFW) of blackberry stem cuttings cv. Tupy treated with different concentrations of indole butyric acid (IBA).
| IBA (mg L-1) | RL (cm) | NR | RFW (g) |
|---|---|---|---|
| 0 | *8.45 ab*** | *2.00 ab*** | *0.36 b*** |
| 500 | 12.14 a | 3.87 a | 0.58 b |
| 1,000 | 2.26 b | 1.00 bc | 0.09 a |
| 2,000 | 0.96 b | 0.08 c | 0.03 ab |
Means followed by the same letters in the column do not differ from each other by Tukey's test at 5% probability. *Observed data; ***Transformed data
According to Dutra et al. (2002) and Hilgert et al. (2020), the application of IBA on woody cuttings during the summer can result in a better-developed root system, both quantitatively - in terms of the percentage of rooted cuttings - and qualitatively, with an increase in the length, number, and fresh and dry weight of roots. In this study, it was found that these parameters were affected by IBA concentrations, with high doses of this hormone impairing the growth and regeneration of new tissue in most tender herbaceous cuttings. This effect was observed in different cultivars of blackberry by Andrade et al. (2007), Dias et al. (2011), and Debner et al. (2019). However, in cuttings of the Xavante cultivar of blackberry, this hormone did not show significant effects, as observed by Maia and Botelho (2008).
Rooting is the most important characteristic in the ultimate survival of plants, with the formation of adventitious roots being the first and foremost step toward successful vegetative propagation (Ahmed et al., 2018). For this to occur, physiological states play a significant role (Gehlot et al., 2015), along with hormonal activity and the benefit of correct concentration and application technique of exogenous growth regulators (Nasir et al., 2021).
According to Druege et al. (2019), the propagation of cuttings becomes highly sensitive to auxin as a key player in the process of root formation, as well as other components considered influencers of root formation through changes in their homeostasis, transport, or signaling pathways mediated by the hormone. Therefore, the need to avoid using growth regulators for root formation is related to the endogenous concentration of auxins as they are found at favorable levels for rooting (Villa et al., 2018), as it must have occurred with the blackberry cuttings in the present study.
Although scientific advancements have been made in the use of plant hormones for the propagation of blackberry stem cuttings with the aim of reducing the difficulty in rooting, it is important to mention that, in order to better utilize cuttings obtained from pruning, it is necessary to apply growth regulators in reduced concentrations. Additionally, when combined with the season of highest temperatures, this can facilitate and accelerate greater cell differentiation of both root and aerial tissues.















