INTRODUCTION
The persistent use of antibiotics has led to several adverse effects, including the emergence of antibiotic-resistant bacteria, suppressed host immunity, microbial population imbalance, and detrimental environmental conditions (Dawood et al., 2022). Biologically active organic compounds have been explored in recent years, leading to the development of natural chemical products derived from plant, animal, and microbial sources. However, natural products derived from plants are viewed as an extensive source of novel chemical compounds with potential medicinal use.
Weed species with inherent abilities to eradicate pathogenic microorganisms have been exploited for their antifungal and antibacterial properties, demonstrating their effectiveness in mitigating fungi and bacteria, reducing environmental impacts. The importance of studying phytochemical composition lies in identifying secondary metabolites with potential biological activity. These metabolites, produced by plant cells through metabolic pathways derived from primary ones, have exhibited various biological effects, including antibiotic, antifungal, and antiviral properties (Chaves-Bedoya et al., 2022).
The genus Melochia, part of the Malvaceae family, encompasses 243 genera and about 4,225 species with a cosmopolitan distribution (Costa et al., 2023). In Colombia, 80 genera comprising 432 species have been identified. Melochia consists of approximately 68 species found in tropical regions and some temperate zones. Around 56 species are reported in America, with Colombia hosting 19 wild-growing species (Baudilio Rondón, 2009). Characterized by a branched stem, simple and alternate leaves, axillary inflorescences of the upper leaves, and dark pink flowers with five petals, this weed is also considered an ornamental resource. However, there are no studies on the vegetative propagation of M. pyramidata through cuttings.
This study explores the antibacterial activity of extracts from Melochia pyramidata, a common weed often overlooked in scientific research, against both Gram-positive bacteria (Bacillus sp., Staphylococcus aureus, and Streptococcus mutans) and Gram-negative bacteria (Klebsiella sp., Escherichia coli, and Serratia sp.). The extract, obtained from freeze-dried plant material, was assessed using the Kirby-Bauer method, offering a measure of the susceptibility of these bacterial strains to the extract (Ortega-Buitrago et al., 2021). The promising results not only underline the untapped potential of weeds like M. pyramidata but also provide significant insights for developing alternative strategies in human healthcare.
MATERIALS AND METHODS
Collection and extraction of Melochia pyramidata L. (purple brush) extract
Samples of M. pyramidata were collected in the municipality of San Jose de Cucuta, Colombia, located at the coordinates 7°56’12” N - 72°29’56” W. The collection site is situated at an elevation of 320 m a.s.l. and experiences a temperature of 34°C.
100 g of plant material, encompassing leaves, stems, flowers, and inflorescences, was utilized for this experiment. The collected sample underwent lyophilization to preserve the components and properties of the plants. Lyophilization, or freeze-drying, allows for the removal of water from plant cells and tissues without altering their structure or the chemical composition of the compounds present. This process yields completely dry plant material.
Following lyophilization, 200 mL of ethanol (Merck, Frankfurt, Germany) was added to the sample, and it was subsequently placed in complete darkness for 24 h in a shaker (MAXQ 4450, Thermo Scientific TM, Marietta, GA) at 35°C and 100 rpm. The ethanol extract obtained was vacuum filtered using filter paper (Qual. Dia. 125 mm, BOECO, Hamburg, Germany) and a vacuum pump (DOSIVAC, Buenos Aires).
The filtered ethanol extract was then concentrated under reduced pressure using a rotary evaporator (IKA® RV10, Wilmington, DE) set at 50 rpm, 150 mbar, and 40°C. The concentrated extract was stored in amber vials at 4°C for subsequent antibacterial analysis against Gram-positive and Gram-negative bacteria.
Identification of secondary metabolites by gas chromatography-mass spectrometry (GC-MS)
The ethanolic extract of M. pyramidata was subjected to analysis using high performance liquid chromatography (HPLC). Chromatographic analysis was performed with an AT6890 Series Plus gas chromatograph (Agilent Technologies, MSD 5973, Santa Clara, CA), which was operating in full scan mode. The column used for the analysis was DB-5MS (5%-phenyl-polymethylsiloxane, 60 m×0.23 mm×0.25 µm). Injection was executed in Split mode (30:1) using the SPME device. The Adams, Wiley, and NIST databases were utilized for this process.
Antimicrobial activity of M. pyramidata extract
The Kirby-Bauer method was employed to evaluate the antimicrobial activity of the ethanolic extract of M. pyramidata. The antimicrobial activity of the ethanolic extract was quantitatively assessed through the presence of inhibition zones and subjected to statistical analysis. Sterile nutrient agar plates were prepared, and sensitized discs (sterilized filter paper) impregnated with 25 μL of the positive control (a selective antibiotic for each bacteria), negative control (distilled water), concentrated extract, and dilutions 1:1, 1:2, 1:3, 1:4, and 1:5 (concentrated extract: ethanol) were placed on the plates. The petri dishes were then incubated under conditions specific to each bacterium for 24 h, and the diameters of the resulting inhibition zones were measured. Statistical analysis was carried out using analysis of variance (ANOVA) and Tukey's multiple comparison tests at a significance level of P<0.05. Each assay was performed three times to ensure statistical validation. For the implementation of these statistical methods, the SAS program was used, providing the necessary tools for an accurate and reliable analysis of the collected data.
RESULTS
Gas chromatography-mass spectrometry (GC-MS)
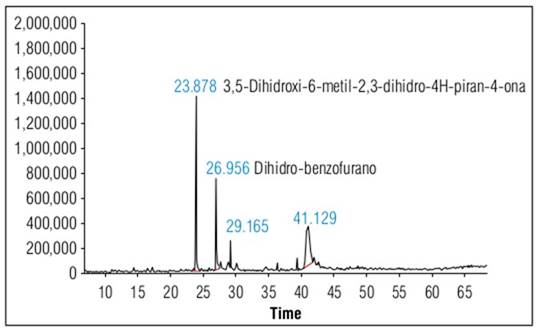
The GC-MS analysis of the ethanol extract of M. pyramidata revealed the presence of various compounds including pyranones, phenolic compounds, terpenes, coumarin, aryl-tetrahydrofurans, and phenylpropanoids. While some of these have been previously reported with various biological properties (Jaramillo-Ordoñez, 2020; Rodríguez-Cepeda and Alvarez-Suarez, 2021), it is important to highlight that the biological activity observed in our assays is attributed to the extract as a whole and not to individual compounds. The results indicate that the M. pyramidata extract exhibits inhibitory activity against microorganisms such as Staphylococcus aureus, Escherichia coli, Bacillus subtilis, and Pseudomonas aeruginosa. These findings suggest a synergistic effect of the compounds present in the extract and highlight the importance of further studies to isolate and evaluate these compounds individually, which will provide a clearer understanding of the mechanisms underlying the observed biological activity.
Table 1 details the compounds identified in our M. pyramidata extracts, along with their relative amounts. It also includes biological activities reported in the literature for these compounds, which were identified in studies other than ours. This distinction clarifies that the biological activities listed are not findings from our current study but are drawn from existing research on these compounds.
Table 1. Primary compounds derived from the ethanolic extract of M. pyramidata.
| Retention time (min) | Compounds | Relative amount GC% (this study) | Biological activity reported* | References |
|---|---|---|---|---|
| 23.97 | 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one | 23.3 | Pseudomona aeruginosa, Staphyloccocus aureus and Candida albicans | Rodríguez-Cepeda and Alvarez-Suarez (2021) |
| 26.56 | Dihydrobenzofuran | 4.2 | Multiple activities reported | Miao et al. (2019) |
| 28.6 | Hydroxyethyl-aniline | 1.4 | - | - |
| 29.93 | 4-Vinylguaiacol | 1.4 | - | - |
| 37.83 | Dihydroactinidiolide | 0.6 | ||
| 40.12 | Hydroxy-β-damascone | 0.7 | ||
| 44.48 | Loliolide | 3.6 | ||
| 48.44 | Palmitic acid | 9.8 | Staphylococcus aureus, Candida albicans and Pseudomonas aeruginosa | Jaramillo-Ordoñez (2020) |
| 48.66 | Hydroxy-methoxy-benzopyranone | 2.9 | ||
| 51.33 | Phytol | 1.9 | Staphylococcus aureus, Candida albicans and Pseudomonas aeruginosa | Jaramillo-Ordoñez (2020) |
| 51.8 | Linoleic acid | 4.3 | Staphylococcus aureus, Candida albicans and Pseudomonas aeruginosa | Jaramillo-Ordoñez (2020) |
| 51.93 | Linoleic acid | 8.1 | ||
| 52.3 | Octadecanoic acid | 0.9 | Staphylococcus aureus, Candida albicans and Pseudomonas aeruginosa | Jaramillo-Ordoñez (2020) |
| 65.3 | Galgravin | 1.8 |
* Includes data from pure compounds and extracts containing them. Blank spaces indicate a lack of specific data for the compound.
Figure 1 depicts the chromatogram of the ethanol extract of M. pyramidata. Several of the compounds identified in M. pyramidata have been attributed with various biological properties. These properties have been linked to several secondary metabolites that contain antibacterial agents effective against microorganisms such as Staphylococcus aureus, Escherichia coli, Bacillus subtilis, and Pseudomonas aeruginosa (Jaramillo-Ordoñez, 2020; Rodríguez-Cepeda and Alvarez-Suarez, 2021).
Effects of the ethanol extract of M. pyramidata on bioassays for Gram-positive and Gram-negative bacteria
The antibacterial activity of ethanolic extract of M. pyramidata was assessed against six bacterial species: Bacillus sp., Staphylococcus aureus, Streptococcus mutans, Klebsiella sp., Escherichia coli, and Serratia sp. Each bacterial species exhibited varying degrees of sensitivity towards the treatments. Bacillus sp. and S. aureus were most responsive to dilution of the concentrated extract 1:4, demonstrating substantial inhibition zones. Meanwhile, S. mutans was effectively inhibited by the dilution 1:5. Klebsiella sp. and E. coli exhibited sensitivity towards multiple treatments, with dilutions 1:3 and 1:4 being the most effective for E. coli. Finally, Serratia sp. was most responsive to dilutions 1:4 and 1:5 (Fig. 2).
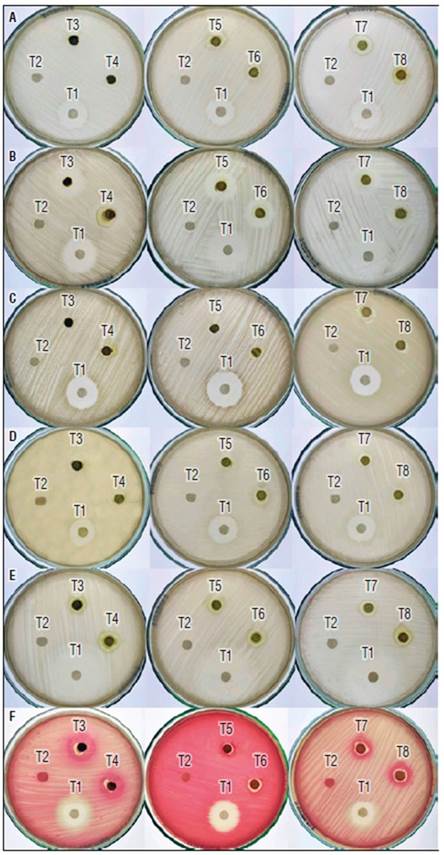
Figure 2. Comparative antibacterial activities of M. pyramidata ethanolic extract dilutions against six bacterial species. The figure contains six panels (A-F), each representing a distinct bacterial species: A) Bacillus sp., B) Staphylococcus aureus, C) Streptococcus mutans, D) Klebsiella sp., E) Escherichia coli, and F) Serratia sp. Each panel presents the results from three petri dishes demonstrating the effects of the different treatments: T1) a specific antibiotic as a positive control, T2) distilled water as a negative control, and T3-T8) various dilutions of the concentrated extract, with T3 being the concentrated extract, and T4-T8 representing dilutions 1:1, 1:2, 1:3, 1:4, and 1:5, respectively.
Statistical analysis and comparative effects of different treatments
The results of the ANOVA showed that the source of variation among treatments was significant, indicating meaningful differences between the treatments in terms of their effect on bacterial growth inhibition. However, the source of variation among replicates was not significant, suggesting that the observed variation in the data was mainly due to differences between treatments rather than replicates. The analysis also indicated that the interaction between bacteria and treatment was significant, revealing differences in the inhibitory effect according to the treatments.
In order to compare the means between groups, an analysis of variance followed by Tukey's test was conducted. The results of Tukey's test revealed significant differences between the means of the groups (P<0.05). The bacterium most sensitive to the different treatments was S. aureus (Gram-positive) with a mean inhibition of 13.12 mm, followed by E. coli (Gram-negative) with a mean inhibition of 10.95 mm. In a third group, the bacteria Bacillus sp. (Gram-positive) and Klebsiella sp. (Gram-negative) displayed average inhibition diameters of 7.75 mm. Finally, the bacteria showing the least response to the different treatments were Serratia sp. (Gram-negative) and S. mutans (Gram-positive). Overall, the ethanol extracts of M. pyramidata showed varying degrees of growth inhibition, depending on the bacterial species and the dilution used. However, it is clear that treatments 1:4 and 1:5 provide the best inhibitory results. These findings align with previous studies that have demonstrated variations in the antimicrobial activity of plant extracts, depending on the bacterial species and the chemical composition of the extracts (Hemeg et al., 2020; Fazeli-Nasab et al., 2021; Alhadad et al., 2022).
DISCUSSION
The compiled data across diverse bacterial strains under various treatment conditions unveils the intricacy and specificity of the antibacterial activity exhibited by the treatments (Tab. 2). This specificity is highlighted by the differential responses elicited in each bacterial species, as observed in the inhibition averages (LSMEAN) and the associated standard deviations (SD). Such variability underscores the nuanced nature of bacterial resistance and susceptibility, emphasizing the critical need for tailored antibacterial strategies. Particularly notable is the consistent ineffectiveness of the negative control across all bacteria, evidenced by a uniform inhibition average of 1 and 0 standard deviation, which validates the experimental design and underscores the specificity of the treatment effects. The response to treatment concentrations ranging from 1:0 to 1:5 show cases a complex pattern of bacterial inhibition, suggesting a dose-dependent relationship for some bacteria but not for others. For example, treatments beyond the control show varying degrees of effectiveness, with certain concentrations leading to significant inhibition in some bacterial strains, while others remain largely unaffected.
Table 2. Antibacterial activity of M. pyramidata ethanol extract: least squares means (LSMEAN), standard deviation (SD), and number of replications (N) by bacteria and treatment.
| Bacteria | Treatment | Inhibition average (LSMEAN) | SD | N |
|---|---|---|---|---|
| Bacillus sp. | Positive control (1) | 18.3 | 0.6 | 3 |
| Negative control (2) | 1 | 0 | 3 | |
| 1:0 (3) | 1 | 0 | 3 | |
| 1:1 (4) | 1 | 0 | 3 | |
| 1:2 (5) | 10.7 | 2.5 | 3 | |
| 1:3 (6) | 9 | 1 | 3 | |
| 1:4 (7) | 11 | 1 | 3 | |
| 1:5 (8) | 10 | 2 | 3 | |
| S. aureus | Positive control (1) | 20.7 | 1.6 | 3 |
| Negative control (2) | 1 | 0 | 3 | |
| 1:0 (3) | 8.7 | 1.6 | 3 | |
| 1:1 (4) | 7.7 | 0.5 | 3 | |
| 1:2 (5) | 16.3 | 3.7 | 3 | |
| 1:3 (6) | 16.3 | 1.2 | 3 | |
| 1:4 (7) | 19.3 | 2 | 3 | |
| 1:5 (8) | 15 | 1 | 3 | |
| S. mutans | Positive control (1) | 19 | 1.7 | 3 |
| Negative control (2) | 1 | 0 | 3 | |
| 1:0 (3) | 1 | 0 | 3 | |
| 1:1 (4) | 1 | 0 | 3 | |
| 1:2 (5) | 1 | 0 | 3 | |
| 1:3 (6) | 1 | 0 | 3 | |
| 1:4 (7) | 1 | 0 | 3 | |
| 1:5 (8) | 10.7 | 0.6 | 3 | |
| Klebsiella sp. | Positive control (1) | 18.3 | 1.1 | 3 |
| negative control (2) | 1 | 0 | 3 | |
| 1:0 (3) | 1 | 0 | 3 | |
| 1:1 (4) | 1 | 0 | 3 | |
| 1:2 (5) | 10.7 | 2.51 | 3 | |
| 1:3 (6) | 9 | 1 | 3 | |
| 1:4 (7) | 11 | 1 | 3 | |
| 1:5 (8) | 10 | 2 | 3 | |
| E. coli | Positive control (1) | 30 | 0 | 3 |
| negative control (2) | 1 | 0 | 3 | |
| 1:0 (3) | 6.3 | 1.1 | 3 | |
| 1:1 (4) | 9.7 | 1.5 | 3 | |
| 1:2 (5) | 9 | 0 | 3 | |
| 1:3 (6) | 11 | 1 | 3 | |
| 1:4 (7) | 11.3 | 2.3 | 3 | |
| 1:5 (8) | 9.3 | 1.5 | 3 | |
| Serratia sp. | Positive control (1) | 14.7 | 0.6 | 3 |
| negative control (2) | 1 | 0 | 3 | |
| 1:0 (3) | 1 | 0 | 3 | |
| 1:1 (4) | 1 | 0 | 3 | |
| 1:2 (5) | 1 | 0 | 3 | |
| 1:3 (6) | 1 | 0 | 3 | |
| 1:4 (7) | 9.7 | 0.6 | 3 | |
| 1:5 (8) | 8.3 | 1.5 | 3 |
The presence of 0 standard deviations in certain treatments reflects the uniformity of response within those experimental replicates, reinforcing the reproducibility of the results. However, this also points to the potential for optimizing treatment concentrations, as some treatments achieve minimal bacterial inhibition, highlighting the importance of adjusting dosages based on the target bacterium (Magréault et al., 2022).
Moreover, the dose-dependent effects observed in specific treatments for certain bacterial strains suggest avenues for further exploration into optimal concentration ranges that maximize inhibitory effects while minimizing potential adverse impacts. The diversity in bacterial response to identical treatment regimens underscores the essential consideration of each bacterium's unique biological characteristics when devising antibacterial approaches (Prajapati et al., 2021).
This study opens new avenues in the quest for novel antimicrobial compounds against bacterial infections. M. pyramidata, considered as a weed, offers potential as a source of bioactive compounds. Identifying the metabolites responsible for their antimicrobial activity could lead to the development of new antimicrobial agents. Reports on the antibacterial properties of M. pyramidata are limited, which is remarkable considering it's often found in abundance as a weed. Moreover, this plant has been associated with cases of intoxication in cattle (Ruíz-Ramírez et al., 2018).
The variability in results observed among different bacterial species and dilutions of the ethanol extract of M. pyramidata can be attributed to the extract's chemical composition and variations in bacterial cell wall structures. Plant extracts have been shown to contain diverse bioactive compounds, including flavonoids, terpenoids, and alkaloids, which possess antimicrobial properties (Othman et al., 2019). Furthermore, the susceptibility of bacteria to antimicrobial compounds can be influenced by the structure and composition of their cell walls, as certain compounds may exhibit a higher affinity for specific cellular structures (Silhavy et al., 2010).
In this study, we found that the positive control, a commercial antibiotic, effectively inhibited bacterial growth in all strains tested, aligning with existing literature demonstrating the efficacy of antibiotics in treating bacterial infections (Stokes et al., 2019). However, the overuse of antibiotics has led to the emergence of multidrug-resistant bacteria (Nikaido, 2009; Serwecińska, 2020), underlining the need for natural alternatives to traditional antibiotics (Rossiter et al., 2017).
The general linear model (GLM) analysis revealed significant outcomes that provide insightful revelations into our study's focal points-the inhibitory diameter effects on different bacteria by varying treatments. The overall model yielded a significance (P<0.0001), affirming the model's robustness in predicting the variable of interest, the diameter of inhibition. This indicates that the model reliably captures the essence of how treatments impact bacterial growth inhibition, laying a strong foundation for inferential interpretations. Particularly, the treatment factor emerged as highly significant, suggesting that the differences among treatments exert a considerable influence on the inhibition diameter (P<0.0001). This implies a clear distinction in the effectiveness of each treatment, with some treatments markedly more potent in inhibiting bacterial growth than others. The significant variance attributed to treatments underscores the necessity to explore these differences further, potentially guiding more effective antibacterial strategies.
On the other hand, the bacterial type also showed significant variance (P=0.0154), indicating variability in bacterial resistance or susceptibility to the treatments. This suggests that the inherent characteristics of each bacterial strain influence their reaction to the treatments. Interestingly, the interaction between replicates and treatments did not manifest significant effects (P=1.0000), suggesting consistency and reliability in the treatment application across replicates. This lends further credibility to the experimental procedure and the replicability of the findings.
However, the interaction between bacteria types and treatments did not yield significant results in this analysis (P=0.2034). This indicates that, while treatments variably affect bacterial growth inhibition, this effect does not significantly differ among the various bacteria tested, under the conditions of this study.
Our results align with previous studies indicating that the antimicrobial activity of plant extracts varies depending on the bacterial species and the chemical composition of the extracts (Elisha et al., 2017; Sharifi-Rad et al., 2017). Additionally, our findings suggest that the ethanol extract of M. pyramidata holds promise as a source of novel antimicrobial compounds for combating bacterial infections.
Ethanol extracts of M. pyramidata have shown promise as natural antimicrobial agents, with several treatments demonstrating significant effects in inhibiting bacterial growth. This suggests the presence of bioactive compounds within the extract capable of combating bacterial infections. Among the identified compounds, 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one was highlighted due to its abundance and potential antibacterial properties (Rodríguez-Cepeda and Alvarez-Suarez, 2021). Given the potential of 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one and other compounds in the extract, future research should explore their mechanisms of action and potential synergies.
It's critical to note that the development of new, safer compounds is a priority in combating bacterial infections. In this context, the study by Miao et al. (2019) becomes relevant as it provides an example of how new derivatives of compounds. It is research emphasizes the ongoing efforts in the pharmaceutical field to create more selective and less harmful antimicrobial agents, reflecting the broader scientific commitment to improving the safety profile of these treatments. Although the major compound identified in our M. pyramidata extract is not directly related to the nitrofurans or the specific derivatives discussed by Miao et al. (2019), the principle of enhancing the therapeutic window of antibacterial compounds by reducing their toxicity remains highly pertinent. This aligns with our conclusion that exploring the bioactive compounds in M. pyramidata for their antibacterial properties, and investigating ways to mitigate potential toxicities, are essential steps forward in the search for new antimicrobial agents.
The significance of this study lies in demonstrating the potential of ethanol extracts of M. pyramidata as natural antimicrobial agents. The identification of new antimicrobial agents is crucial, given the growing and urgent public health problem of antibiotic resistance worldwide. Moreover, the concerns regarding the side effects of antibiotics, such as alterations to the intestinal microbiota, underscore the importance of exploring natural alternatives to traditional antibiotics (Piddock, 2012).
Furthermore, several justifications support conducting experiments to evaluate the antimicrobial activity of plant extracts, as in the case of this study assessing the ethanol extract of M. pyramidata. Firstly, antibiotic resistance is an escalating global problem, necessitating the need for natural and sustainable alternatives to combat bacterial infections (Helmy et al., 2023). Plant extracts have been employed in traditional medicine for centuries, and research in this field can contribute to the development of new antimicrobial compounds (Cowan, 1999). Secondly, the search for new antimicrobial compounds is particularly critical in the case of multidrug-resistant (MDR) bacterial infections (Terreni et al., 2021). These infections pose a serious threat to public health as they do not respond to conventional antibiotic treatments.
Developing novel antimicrobial compounds can help address MDR infections and mitigate their impact on public health (Miethke et al., 2021). Plants, and importantly often overlooked weeds like M. pyramidata, offer a potentially rich and largely untapped source of bioactive compounds with diverse health benefits. Weeds, traditionally seen as nuisances in agriculture, could instead provide significant value. Their robust nature, ability to grow in various conditions, and bioactive properties could transform them into potential goldmines for biocompounds. This transformation could bring a new perspective for farmers and agriculturalists, possibly turning problematic weeds into beneficial crops. Incorporating the cultivation of such weeds into farming practices could lead to dual benefits. On one hand, farmers could profit from a new, resilient crop; on the other, this would help support the development of novel antimicrobial compounds.
Evaluating the antimicrobial activity of plant extracts not only aids in the development of new antimicrobial compounds but also facilitates the discovery of other bioactive properties within the extracts (Chaves-Bedoya et al., 2022). With such potential benefits, a more integrative approach, combining agriculture and drug discovery, could be a groundbreaking path for future research. This integration could bridge the gap between agricultural practices and healthcare innovations, bringing a new dimension to farming while providing society with natural, sustainable, and accessible solutions to combat antibiotic resistance.
While highlighting the antimicrobial potential of M. pyramidata, it is important to acknowledge certain limitations. The study focuses on the ethanolic extract, which may not represent the full spectrum of bioactive compounds present in the plant, and the complexity of the extract as a mixture makes it challenging to attribute the observed activity to any specific compound. Natural variability in the plant's composition due to environmental factors could affect the reproducibility of results (Pacheco-Hernández et al., 2021). Additionally, comprehensive toxicological studies are required to understand the safety profile of the plant extract for therapeutic use. Translating these findings to practical applications presents challenges in large-scale production and standardization, and the potential for resistance development over time needs to be evaluated for long-term effectiveness.
CONCLUSION
This study has unveiled that the ethanolic extract of Melochia pyramidata L., commonly underestimated as a weed, harbors antibacterial potential, opening new avenues for the development of antimicrobial alternatives amid the escalating antibiotic resistance crisis.
The significant capability of the extract to inhibit growth in both Gram-positive and Gram-negative bacteria, notably against S. aureus and E. coli, underscores its relevance in the search for new compounds with antimicrobial activity. Through a rigorous methodological approach, this study has not only quantified the antibacterial activity of the extract but also identified specific compounds, such as 3,5-Dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one and Dihydrobenzofuran, which might be partly responsible for this activity.
Furthermore, the research emphasizes the importance of exploring unconventional natural sources, like weeds, historically undervalued in the pharmacological context. This suggests a reevaluation of plant biodiversity and its potential to address global health challenges. The efficacy of M. pyramidata extract against resistant pathogens highlights the urgency of integrating multidisciplinary approaches that combine phytochemistry, microbiology, and pharmacology to develop innovative strategies to tackle antibiotic resistance.
Facing the critical need for new antimicrobial solutions, the findings of this study not only expand our understanding of the antibacterial properties of M. pyramidata but also establish a solid foundation for future research focused on the clinical validation of these extracts or isolated compounds. It is imperative that future research directs towards evaluating the in vivo efficacy, safety, and mechanism of action of the identified compounds, which could eventually lead to the development of new antimicrobial agents for medical use.
By providing tangible evidence of the antibacterial capacity of extracts derived from M. pyramidata and highlighting the relevance of investigating less conventional natural resources, this study makes a significant contribution to the field of antimicrobial research, offering new perspectives and tools to combat antibiotic resistance.